Type species: Sphinx euphorbiae Linnaeus, 1758.
A cosmopolitan genus containing up to 29 species and 42 subspecies (Kitching & Cadiou, 2000), depending on how one classifies the populations and races. Hyles appears to have evolved in the Neotropics during the Oligocene/Eocene epochs. It is a very young genus that radiated on a global scale rather quickly (Hundsdörfer et al., 2005b).
(Taxonomic notes. (i) Within the Hyles genus there is a complex of species, subspecies and forms, all closely related to Hyles euphorbiae (Hundsdörfer et al., 2005b), and all of which are highly polymorphic with an amazing variety of colour forms, some geographic in nature, others environmental. The Hyles euphorbiae complex (HEC) is rather difficult to classify for it would seem to be in the process of diverging into a number of species. Many of the following species and subspecies are regarded by some authors (e.g. Speidel & Hassler, 1989) simply as subspecies and forms, respectively, of Hyles euphorbiae. Full mtDNA barcoding of this complex is underway but has yet to be completed. When completed it may solve some of the remaining relationship problems as it has for the Papilio (Pterourus) glaucus (Linnaeus) complex in North America (Hagen et al., 1991; Hagen & Scriber, 1989, 1991). Examination of the genitalia is of little use as they show practically no difference between taxa; however, the mtDNA studies by Hundsdörfer et al. (2005b; 2011b) has started to unravel some inter- and intraspecific relationships.
Larval coloration and pattern do, however, provide a very good guide to the relationships between the many 'species' and 'subspecies'; this same conclusion was also reached by Shchetkin (1956) and Meerman (1993). By reference to these, in combination with other features, such as differences in female 'calling' times (Harbich, 1994), it is possible to group together certain species, subspecies and forms. However, it should be noted that individuals with very divergent haplotypes can have very similar larval morphology, and larvae with very different combinations of pattern elements can have identical or closely connected haplotypes (Hundsdörfer, Mende, Kitching & Cordellier, 2011). Sometimes it can be difficult to assign a population to one taxon or another.
(A numerical taxonomic analysis (using phenograms) of this complex by Meerman (1993) broadly supports these groupings, as do the studies of Hundsdörfer et al. (2005b).)
An intriguing explanation for the variability of Hyles euphorbiae s. str. has been put forward by Harbich (1975; 1976a). Primary hybrids between Hyles euphorbiae s. str. and other Hyles species were able to produce viable offspring when back-crossed with Hyles euphorbiae s. str., but not, however, with the other parent. Thus the variability of Hyles euphorbiae s. str. could be the result of a genetic influx from other members of the genus, such as Hyles hippophaes (Esper, 1789), Hyles chamyla (Denso, 1913), Hyles vespertilio (Esper, 1780) and Hyles gallii (Rottemburg, 1775) (see Harbich, 1976c), which themselves would be protected from the influence of Hyles euphorbiae. This view is supported to some extent by the findings of Hundsdörfer et al. (2005b). Of course, the degree of influence the other species have on Hyles euphorbiae would depend on how often interspecific crosses occur, which, in turn, depends on how closely related the species are. Additionally, gene flow between Hyles tithymali (Boisduval, 1834) and Hyles euphorbiae may be decreased by genetic linkage between ecologically important traits in sex chromosomes, such as an adaptation or lack of adaptation to desertic conditions. These species appear to be primarily maintained by ecological selection reinforced by behavioural differences, and not genetic barriers. (For more information see Harbich (1988, 1989, 1991, 1992, 1994).
Care should be taken when investigating the biogeography and evolution of populations of this complex based on mtDNA. The present day pattern of geographically separated mitochondrial lineages is commonly interpreted as being the result of long term evolutionary processes. However, the results of Mende & Hundsdörfer (2013) indicate that new patterns can emerge surprisingly quickly in the Hyles euphorbiae complex. They caution against drawing hasty taxonomic conclusions based solely on modern-day spatial patterns of mitochondrial markers.
At the beginning of the twentieth century the European mainland lineages were present at a moderate frequency in southern Italy and Sicily. The proportion of an 'italica' lineage then steadily increased in this area from below 60% to near 100% in about 120 years. This indicates that in Italy the historical geographical distribution of mitochondrial lineages in the Hyles euphorbiae complex was different to what current demography suggests. The modern-day pattern of an integral 'italica' core region and a disjunct 'euphorbiae' distribution is a very recent phenomenon. To explain these strong demographic changes, Mende & Hundsdörfer (2013) proposed genetic drift due to anthropogenic habitat loss and fragmentation in combination with the impact of recent climate change, which favoured the spread of the potentially better adapted 'italica' populations.
A more in-depth genetic analysis was undertaken by Mende, Bartel & Hundsdörfer (2016) to try and unravel further the relationships within the Hyles euphorbiae complex (HEC). Three mitochondrial genes were amplified using the primers given by Hundsdörfer, Kitching & Wink (2005b; 2005c). This was combined with an analysis of six microsatellite loci as described by Mende, Stuckas & Hundsdörfer (2011), and another six loci as developed by Hundsdörfer, Sanetra, Corbeil & Stuckas (2009).
MITOCHONDRIAL ANALYSIS: Network analysis of mitochondrial sequences corroborated the previously found division of the HEC into seven haplogroups. Additionally, a specimen each from Pantelleria Island (Strait of Sicily) and northern Tunisia were found to carry the distinct Sardo-Corso-Balearic endemic Hyles dahlii haplotype. Three haplogroups were detected in the entire Eurasian range of 'pure' Hyles euphorbiae, namely 'euphorbiae', the less frequent 'enigmatica' and the very distinct 'melitensis'; none of the three occurs in the range of the Afro-Macaronesian Hyles tithymali. However, 'enigmatica' appears to be connected to the 'tithymali' haplogroup via the eastern 'robertsi'. This haplogroup is restricted to Iran, where it is mixed with 'euphorbiae' and 'enigmatica' to give the 'species' Hyles robertsi (according to some taxonomies). In contrast, the range of Hyles tithymali and continental North African putative hybrid populations is predominantly occupied by a single haplogroup -- 'tithymali'. This is absent from the range of morphologically 'pure' Hyles euphorbiae; its sole occurrence on the European mainland is in the putative hybrid populations of Central Italy.
Populations considered to be of hybrid origin based on their larval morphology carried remarkably different mixes of mitochondrial haplogroups. The north-western Spanish population was found to contain only 'euphorbiae' haplotypes, which contradicts its taxonomic status as Hyles tithymali gallaeci. Malta was confirmed as consisting of a mixture of haplotypes belonging to 'tithymali' and two haplogroups, namely 'euphorbiae' and 'melitensis', that also occur in mainland Hyles euphorbiae. In contrast, the other Mediterranean putative hybrid populations were dominated by haplogroups that are equally closely related to 'tithymali' and each other -- the Apennine Peninsula, Sicily, and Pantelleria by 'italica', and the southern Aegean Islands by the endemic 'cretica'. In addition, 'italica' occurred more frequently in the North African populations than was previously found. Additionally, 'italica' was also found in the supposed non-hybrid Hyles tithymali deserticola of southern Morocco. A few individuals bearing 'italica' haplotypes were also detected in areas of Mediterranean Europe assigned to morphologically 'pure' Hyles euphorbiae. In turn, the Hyles euphorbiae haplogroups 'euphorbiae', 'enigmatica' and 'melitensis', were found at low levels in the dominant 'italica' of Southern Italy.
MICROSATELLITE LOCI: Nuclear genetic and geographic distances between populations are significantly correlated across the HEC's entire range. Accordingly, 18.3% of the genetic divergence can be explained by geographic distance. Results of a hierarchical AMOVA (average over 12 loci) indicated that 9.9% of the total variability in the dataset was found between the two groups of populations defined a priori, supporting the division of the western HEC into Hyles euphorbiae and Hyles tithymali. The geographic ranges of the two clusters were largely congruent with the traditionally defined 'pure' ranges of these two main taxa. However, at least a few individuals with admixed ancestry are recorded throughout most parts of both the species' ranges. Nearly all individuals bearing 'tithymali' haplogroup mitochondria can be assigned to the Hyles tithymali microsatellite cluster, whereas individuals bearing all other haplogroups are assigned to the Hyles euphorbiae cluster. Accordingly, the two microsatellite clusters both contain a high number of individuals with private haplotypes. The Yemeni Hyles tithymali himyarensis represents an unexpected exception to the congruence between microsatellite clusters and 'pure' taxa ranges, since they fall predominantly into the Hyles euphorbiae microsatellite cluster by both cluster analyses: this conflicts with their morphological and mitochondrial affiliations. Likewise, the assignment of individuals of the Iranian Hyles robertsi to the Hyles euphorbiae cluster conflicts with their partly 'tithymali'-related 'robertsi' haplogroup mitochondria. Remarkably, the diversity of both nuclear and mitochondrial markers is distinctly higher in the Hyles euphorbiae than in the Hyles tithymali cluster. Thus, the geographic position of the main divide between the two microsatellite clusters runs along the Mediterranean putative hybrid populations.
To sum up, analyses of the nuclear microsatellite data concordantly supported a superordinate division of the Western Palaearctic HEC into two clusters. These roughly correspond to the two traditionally defined species, the Eurasian Hyles euphorbiae and Afro-Macaronesian Hyles tithymali. The mitochondrial haplogroups 'enigmatica', 'robertsi', 'italica' and 'cretica' are almost exclusively restricted to individuals in the microsatellite cluster representing Hyles euphorbiae (and the distribution range enclosed by these samples), whereas they are more closely connected to the main haplogroup of Hyles tithymali in the network of mitochondrial sequences ('tithymali'). This discordance demonstrates an incomplete separation of Hyles euphorbiae and Hyles tithymali.
DISCUSSION: Cumulative evidence points to the HEC consisting of only a single gene pool, though structured into two main clusters. The populations studied could thus be classified as belonging to a single species according to the classical biological species concept (Hundsdörfer, Lee, Kitching & Mutanen, 2019). Although a division into two clusters corresponding to Hyles euphorbiae and Hyles tithymali is well supported by the microsatellite data, Hyles euphorbiae is not a maternal lineage monophylum (mtDNA), in the sense that the taxon does not contain all descendants of its most recent common ancestor. It was found that mitochondrial material from Hyles dahlii was also present. Individuals of Hyles dahlii (the HEC species endemic to Sardinia, Corsica and the Balearic Islands) have been reported sporadically from the surrounding mainland coasts and interpreted as rare dispersal events. However, the detection of individuals carrying a Hyles dahlii haplotype in the established HEC populations suggests that dispersal across the Mediterranean Sea and subsequent hybridisation may be more common than presumed. These events probably contribute to the morphological variability in the HEC. The 'enigmatica' haplogroup is currently most frequent in some Italian and Central European populations, but has no core area. An augmented dataset further revealed that the relic 'melitensis', which is highly divergent from 'euphorbiae', is not endemic to Malta as previously assumed, but also occurs on Sicily and the European mainland.
In contrast, the morphology of adult Yemeni specimens strongly resembles Hyles tithymali, whereas their predominant nuclear genetic assignment is to Hyles euphorbiae. The adjacent Saudi Arabian population has morphologically been assigned to Hyles euphorbiae, but also features 'tithymali' mitochondria, indicating a complex admixture pattern in this region. However, a considerably larger sample size is needed for a more robust assessment of the population genetic structure and its history in this region.
The Galician population (NW Spain and Portugal) had also been considered a hybrid population based on morphology. However, no trace of Hyles tithymali was found in either molecular marker, which contradicts what the larval colour patterns indicate. This may be due to the low sample size and the already low differentiation of the two microsatellite clusters.
The observed genetic admixture between Hyles tithymali and Hyles euphorbiae and the high dispersal potential of the HEC lead Mende, Bartel & Hundsdörfer (2016) to postulate a broad trans-Mediterranean 'admixture belt' that repeatedly oscillated north and south with climate changes resulting a mix of expanding and contracting hybrid populations. These post-glacial zones/belts of secondary contact and hybridization between once isolated sister species are more commonly known as 'suture zones', and have been reported for a variety of taxa in many biomes (Barrowclough et al., 2019; Ipekdal et al., 2020; Moritz et al., 2009; Portnoy & Gold, 2012).
Indeed, a locally varying interplay of geographic barriers and climate variables, as well as incomplete lineage sorting and introgression, appear to have shaped the truly complex phylogeography of this dispersive hawkmoth species complex. The dynamic effects of this on indentifiable species, subspecies, sibling species, extinction and adsorption has been commented on by Arce-Valdés & Sánchez-Guillén (2022) in this species pair as well as other insects. This, and the work of Hundsdörfer, Lee, Kitching & Mutanen (2019), has highlighted the importance of comprehensive temporal sampling and analyses of multiple markers for a conclusive evaluation of a species' evolutionary history. The data implied that the populations studied constitute a single gene pool, and thus belong to one biological species (Hundsdörfer, Lee, Kitching & Mutanen, 2019). Nevertheless, a division into two distinct subgroups is very evident, so-much-so, that for the purposes of this work the HEC subgroups 'tithymali' and 'euphorbiae' are treated as two separate but closely related species which have yet to fully speciate, i.e. they are sibling species.
This division is supported by the discovery of higher cold sensitivity in the pupae of Hyles tithymali s. str. populations, which exhibit rather opportunistic and short-term cold hardiness, whereas Hyles euphorbiae s. str. produces a phenotype with seasonal cold-hardy diapause pupae under the combined effect of short daylight length and continuous cold treatment. Further differences include variability in the duration and mortality of diapause pupae. This suggests different pre-adaptations to seasonal environmental conditions in each 'ecotype', indicating incipient speciation within the Hyles euphorbiae complex (Daneck, Barth, Geck & Hundsdörfer, 2021).
This incipient speciation is further supported by a recent phylogenetic reconstruction of the genus Hyles utilizing the extended barcode method. Hyles tithymali, Hyles euphorbiae and the Asian Hyles costata (Nordmann, 1851) cluster together in their own clade, but as distinct and separate entities. In other words, the three taxa are confirmed as distinct but related species within the genus Hyles.)
(ii) Hyles costata (Nordmann, 1851), found from the Altai through Mongolia to Amurland and northern China (Derzhavets, 1979a), is a valid species with very distinct larvae and hostplants. It appears to be related to Hyles tithymali (Hundsdörfer et al., 2005b). However, Meerman (1993) considered Hyles costata to be a subspecies of Hyles euphorbiae, although somewhat distant.
(iii) Hyles exilis Derzhavets, 1979, also found from the Altai to northern China, is also a valid species, but with larvae very similar to those of Hyles euphorbiae conspicua (Rothschild & Jordan, 1903). Only extensive mtDNA studies will determine just how close this Euphorbia-feeding species is to Hyles euphorbiae.)
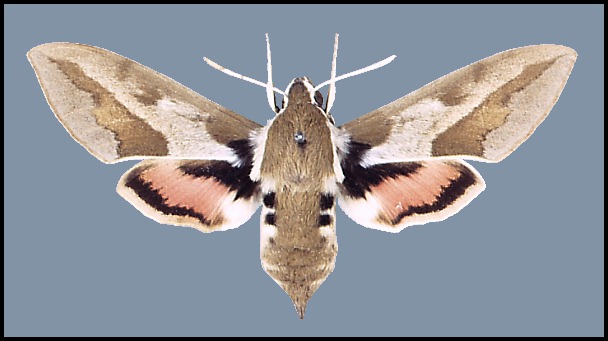
IMAGO: Forewing entire with apex pointed. Forewing pattern very distinctive, consisting of a pale cream or white, oblique median stripe in most species. Antenna distally incrassate in both sexes and, in female, strongly clavate. Pilifer with long setae medially, short setae laterally. Eye lashed. Labial palpus smoothly scaled with no erect hairs, concealing base of proboscis; scaling at apex of first segment not arranged in a regular border on inner surface; second segment without apical tuft on inner side. Abdomen conically pointed, with strong dorsal spines usually arranged in three rows. External spines of foretarsus more or less prolonged, always longer than the respective spines on the inner side of tarsus; comb of mid- and hindtarsus vestigial, the spines not much prolonged. First segment of hindtarsus shorter than tibia. Inner distal spur of hindtibia more than twice as long as outer and about half tibial length. Pulvillus absent or vestigial.
Genitalia. Very simple and similar in all species, thus of little use in distinguishing species. In male, valva broadly sole-shaped; friction scales numerous and small in most species. Sacculus ending in a thin, more or less curved, simple, tapering process. Phallus with dorsal apical edge incrassate, dentate, produced at left side into a short process; length of brim-like incrassation, as well as dentation, slightly different in various species.
Behaviour. An interesting characteristic of all Hyles species is their reaction to disturbance. Dropping to the ground, the abdomen is hunched, the wings are swung upwards and the antennae laid forward until they touch. The insect then hops about in short jumps.
OVUM: Small, almost spherical, pale green or blue-green.
LARVA: Typically sphingiform; thorax slightly tapering anteriorly. Usually with a conspicuous dorso-lateral line of eye-spots. Many are polymorphic, the forms themselves varying in background colour, spots and the colour of these spots. Horn present or absent.
PUPA: Proboscis fused with body, not projecting anteriorly, not obviously carinate. Light tan, smooth, covered with short, fine dark lines. Cremaster large, sometimes bent ventrally, with an apical pair of spines. In a fairly substantial cocoon on the ground.
HOSTPLANT FAMILIES: Principally herbaceous plants of the Euphorbiaceae, Onagraceae, Rubiaceae, Zygophyllaceae, Elaeagnaceae and Xanthorrhoeaceae/Asphodelaceae.

UK: Spurge Hawkmoth; Leafy Spurge Hawkmoth, F: Sphinx de l'Euphorbe, D: Wolfsmilchschwärmer, RUS: Molochainyi Brazhnik, S: Vitsprötad Skymningssvämare, NL: Wolfsmelkpijlstaart, CZ: Lišaj pryšcový, H: kutyatejszender, E: esfinge de la lechetrezna, PL: Zmrocznik wilczomleczek, FIN: Tyräkkikiitäjä, I: sfinge dell'euforbia, HR: mlječikin ljiljak, DK: Vortemælksværmer, EST: Piimalille-vöötsuru.
Sphinx euphorbiae Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 492.Type locality: Unspecified [Europe].
(Taxonomic notes. (i) 'Subspecies' strasillai, rothschildi, subiacensis, dolomiticola and filapjewi are all forms which were incorrectly given subspecific status; they can be found intermittently throughout the range of Hyles euphorbiae euphorbiae. 'Subsp.' lucida does not warrant subspecific status as it falls within the normal variation of Hyles euphorbiae euphorbiae, both as a larva and adult.
(ii) Meerman (1993) disagrees with the inclusion of Hyles euphorbiae vandalusica within Hyles euphorbiae euphorbiae and gives this form full subspecific status based on phenographic analysis.
(iii) It should be noted that some populations in northern Portugal and northwestern Spain appear to be hybrids of this subspecies and Hyles tithymali gecki De Freina, 1991, similar to those found on Malta (Hundsdörfer, Mende, Harbich, Pittaway & Kitching, 2011). The larvae range in colour and patterning from typical Hyles tithymali gecki to typical Hyles tithymali 'sammuti' Eitschberger, Danner & Surholt, 1998; the adults are very much like Hyles euphorbiae euphorbiae f. vandalusica from southern Portugal and southern Spain [see below for adult and larva], with a touch of tithymali in some. They are thus similar to the adults of the proven hybrid Hyles tithymali 'sammuti', but less red. The settlement and spread pattern of this recent hybrid population indicate landfall along the coast by a few migrant adults (most likely males) riding the prevailing winds from Madeira, and interbreeding with the resident Hyles euphorbiae euphorbiae. However, Gil-T., Requejo & Estévez (2011) dispute this and claim that this 'little known' population (which has been known about for several years) is a post-ice age relict, giving it the name Hyles tithymali gallaeci. Legs of adults have been sent for mtDNA analysis, which should settle this divergence of opinion. Initial results have already confirmed that the maternal ancestor of the Minho population (Portugal) was Hyles euphorbiae euphorbiae (Hundsdörfer, Mende, Kitching & Cordellier, 2011).)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]



Holarctic; western Palaearctic region. Pleistocene refuge: Polycentric -- Atlantomediterranean, Adriatomediterranean and Pontomediterranean subsections of the Mediterranean refuge, and the Caspian and Iranian refugia. Probably also several of the adjacent eremic refugia.


Wingspan: 70--85mm. Very similar to the other subspecies and to Hyles tithymali, Hyles centralasiae (Staudinger, 1887) and Hyles nicaea (de Prunner, 1798). Normal specimens of each are illustrated in Pittaway (1993) but, due to the great wing-pattern variation exhibited by Hyles euphorbiae euphorbiae, some confusion may arise. Such variation includes wings with no trace of red; wings deeply flushed with red (f. grentzenbergi Staudinger); hindwings rust-brown (f. brunnescens Schultz), or yellow (f. lafitolei Thierry-Mieg) in place of the normal red. All other coloration can vary widely, while in the most extreme form the entire forewing is brown with a series of small, cream, dagger-like markings replacing the oblique median stripe (f. restricta Jordan).



Many Chinese individuals are more heavily spotted in a manner similar to Hyles nicaea sheljuzkoi Dublitzky, 1928 (Pittaway, pers. obs.).




Can be very common in warm, dry years but very scarce in cold, wet ones. Found where perennial herbaceous Euphorbia species are present, with open, sunny locations being preferred, such as along the edges of fields and woodland, on coastal sand-dunes and on mountain-sides up to 1900m in the Alps.

By day, most individuals rest on stones, low walls, amongst low vegetation, or even on the ground, taking flight just after dusk for a short period and again later, in three or four bouts of activity which, in the males at least, usually cease by midnight. It is during one of these that pairing takes place, usually four to five days after emergence and usually between 22.00 and 24.00 hours (Harbich, 1994), although Friedrich (1975, 1982, 1986) quotes 21.00 -- 23.00 hours. The pair remain in copula for up to three hours. Following separation, females begin oviposition almost immediately, laying a few eggs on the first night and more on subsequent nights. Although this species is attracted to flowers and feeds avidly from beds of, for example, Petunia, Oenothera, Echium, Epilobium, Nicotiana or Silene, few females come to light.
If threatened and feeling at risk, resting adults will drop to the ground, raise their wings, press their antennae together frontad, arch their bodies, run and hop around, while flicking their forewings back and fourth to expose the red patch on the hindwings. Although not so extreme, this behaviour is also seen in many other sphingidae, especially Sphinx ligustri Linnaeus, 1758 and other members of the genus Hyles.


Univoltine in its northern range, mainly from mid-June to the beginning of July, although individuals can be found as early as mid-May. Farther south, two generations are normal, one in May/June, the other in August/September. However, at higher altitudes in southern Europe there is only one generation during June and July (Ganev, 1984). In the southern Urals, from late May until early July, late July, with a partial second/third generation in late August--early September (Nupponen & Fibiger, 2002).
OVUM: Almost spherical (1.1 x 1.0mm), light glossy blue-green. Deposited either singly or in small clusters at the tip of a shoot of the hostplant, hatching about ten days later. Interestingly, the eggs of this species are extremely hard; if dropped on to a hard surface they will rebound to a considerable height.

LARVA: Full-fed, 70--80mm. Polymorphic.


The newly-hatched larva measures approximately 4mm and is off-white with a black head and horn. With feeding, this primary colour is soon replaced by dark olive black which then lightens with further feeding. At this stage it rests low down on the hostplant during the day, at dusk moving up the stem to feed during darkness in the company of others. After the first moult, the characteristic bright pattern appears, superimposed on a now light greenish yellow-brown background.


With each successive moult this pattern becomes more startling until, eventually, the fully-grown larva can feed quite openly, relying on its aposematic coloration for protection. Although the basic pattern remains the same, size and colour are just as variable in the larva as in the adult insect (see Buckler, 1887). The dorso-lateral line of eye-spots may be red, yellow, green, white or orange, set in a ground colour which varies from green through light yellowish brown to black.



In all stages, larval feeding powers are prodigious. Vast quantities of leaves and also soft stems are consumed between spells of basking, which it does more frequently as it grows. If disturbed, a thick stream of dark green fluid is ejected from the mouth towards the attacker, accompanied by violent body twitching from side to side. Hundsdörfer, Tshibangu, Wetterauer & Wink (2005) have demonstrated that although this species does not sequester plant toxins in its body tissues, the green plant slurry present in the anterior gut is rich in phorbol esters, which are potent toxins and irritants. Protection by toxic gut contents (and not sequestered toxins) is also found in the larvae of Daphnis nerii (Linnaeus, 1758).




Prior to pupation, the larva is very active and wanders a great distance at a very fast pace, finally pupating a considerable way from the hostplant.
The larvae are very sensitive to photoperiod and temperature, and will not go into diapause if exposed to long-day conditions of 17 hours light and 7 hours darkness and >18°C (Harbich, 1976b).
Occurs in most areas throughout July and August; in June and August/September where two generations are present.

Major Hostplants. Herbaceous species of Euphorbia, especially Euphorbia paralias, Euphorbia cyparissias and, in southern Europe, Euphorbia characias.
Minor Hostplants. Very occasionally, Rumex, Polygonum, Vitis, Mercurialis and Epilobium. In Switzerland it is not infrequently found on Polygonum aviculare (Schweizerischer Bund für Naturschutz, 1997).

PUPA: 45--50mm. Light yellowish brown with fine, dark striations. Formed within a loosely spun, net-like cocoon, incorporating leaves, soil and other debris, on the surface of the soil. The time spent in this stage can vary considerably -- from two weeks to two years. Overwinters as pupa.



Braconidae: Cotesia glomerata (Linnaeus, 1758) [syn. Cotesia nigriventris (Nees, 1834)], Cotesia saltator (Thunberg, 1822); Tachinidae: Blepharipa pratensis (Meigen, 1824), Blondelia nigripes (Fallén, 1810), Compsilura concinnata (Meigen, 1824), Drino (Zygobothria) atropivora (Robineau-Desvoidy, 1830), Drino galii (Brauer & Bergenstamm, 1891), Drino vicina (Zetterstedt, 1849), Exorista grandis (Zetterstedt, 1844), Exorista larvarum (Linnaeus, 1758), Masicera sphingivora (Robineau-Desvoidy, 1830), Pales pavida (Meigen, 1824), Pales processioneae (Ratzeburg, 1840), Phryxe erythrostoma (Hartig, 1838), Winthemia cruentata (Rondani, 1859), Winthemia quadripustulata (Fabricius, 1794).

From western Europe (Portugal (Corley, 2004)), and southern and central Europe to the Urals (Kumakov, 1977; Nupponen & Fibiger, 2002), southern western Siberia (Zolotarenko, Petrova & Shiryaev, 1978; Izerskiy, 1999; Dubatolov, pers. comm. 2010; Knyazev, 2020; Maksimov et al., 2022), the western and southern fringes of the Altai Mountains (Izerskiy, 1999; Saldaitis & Ivinskis, 2006) and western China (Xinjiang Province (Pittaway & Kitching, 2000)). Also, northern Turkey (Daniel, 1939; de Freina, 1979), the Republic of Georgia, Armenia and Azerbaijan (Didmanidze, Petrov & Zolotuhin, 2013; Streltzov et al., 2022), southeastern Kazakhstan (Shovkoon, 2015; Toropov, Milko, Zhdanko & Evdoshenko, 2023), eastern Uzbekistan (Shermatov et al., 2021; Toropov, Milko, Zhdanko & Evdoshenko, 2023; Omonov, Rahimov, Askarova & Khomidova, 2023); Kyrgyzstan (S. Toropov, pers. comm.; Korb, 2018; Toropov, Milko, Zhdanko & Evdoshenko, 2023), northeastern Afghanistan (Ebert, 1969), northern Pakistan (Rafi et al., 2014), and the western Tian Shan (Alpheraky, 1882; Derzhavets, 1980). Not found in Corsica, Sardinia or the Balearic Islands (Pittaway, 1983b). A migrant to the north.
The eastern populations from the Pamirs, eastern Afghanistan and Kashmir (subsp. orientalis Ebert, 1969 = subsp. elisabethae Ebert, 1996) were once regarded as a separate subspecies of Hyles robertsi (Butler, 1880), although they now appear to be a mixture of hybrids with Hyles euphorbiae euphorbiae, including f. peplidis Christoph, 1894.
It should be noted that some populations in northern Portugal and northwestern Spain appear to be hybrids of this species and Hyles tithymali gecki.
Extra-limital range. The eastern Tian Shan (Bang-Haas, 1936), much of Xinjiang Province, China (Pittaway & Kitching, 2000), and southwestern Mongolia (Saldaitis & Ivinskis, 2006).
Also, introduced into many areas of the U.S.A. and Canada to control non-native pest species of Euphorbia (Hodges, 1971; New, 1971; Forwood, 1977; Batra, 1983; Winston et al., 2014). It is particularly common in Montana, Alberta and Ontario, and has spread as far west as Spokane, Washington State (Carl Barrentine, pers. comm. 2022).
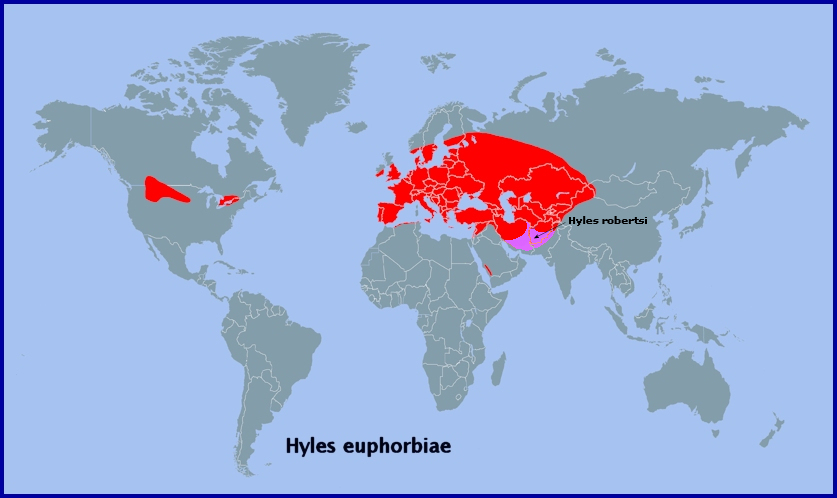
Israel, Jordan, Lebanon, Syria, southern Turkey, northern Iraq, northern Iran, the Kopet-Dagh Mountains of Turkmenistan and Iran, and into Uzbekistan, as subsp. Hyles euphorbiae conspicua (Rothschild & Jordan, 1903), with an isolated population in the south-western mountains of Saudi Arabia.
Farther east, in the bulk of the Altai, southern Siberia, Transbaikalia, Mongolia and north-eastern China, it is replaced by the related Hyles exilis Derzhavets, 1979.
 Return to species list
Return to species list