Type species: Sphinx nerii Linnaeus, 1758.
A genus of the Old World tropics and subtropics, containing seven to nine species.
IMAGO: Wing entire in outline, with forewing apex pointed. Usually some shade of green or brown with curved bands and triangles of different colours. Eye large, without pendant lashes. Labial palpus large, obtuse, smoothly scaled. Antenna slightly clubbed in female; setiform in male, with a long thin terminal segment. Spines of abdomen in several rows, elongate, weak; first tergite large. Tibiae without spines. Midtarsus with basal comb. Hindtibia with two pairs of spurs, the proximal being considerably longer than the distal. M2 of hindwing before centre of cell.
Genitalia. In male, valva with fewer than ten large, erect, modified friction scales on outer surface. Sacculus with two processes, one proximal, the other distal, both dorsal. Phallus with one or two left processes, and a longer right process. In the female, lamella postvaginalis suddenly narrowed at end where it is concave; apical margin raised and projecting ventrad.
OVUM: Ovoid, pale green, shiny.
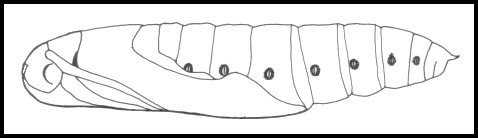
LARVA: Head small, body tapering sharply forward from abdominal segment 1, rest of body almost cylindrical. Usually bears a prominent eye-spot on its third thoracic segment and a pale dorso-lateral line from abdominal segment 1 to the horn. Horn stout and curved downward in final instar. Body smooth in final instar.
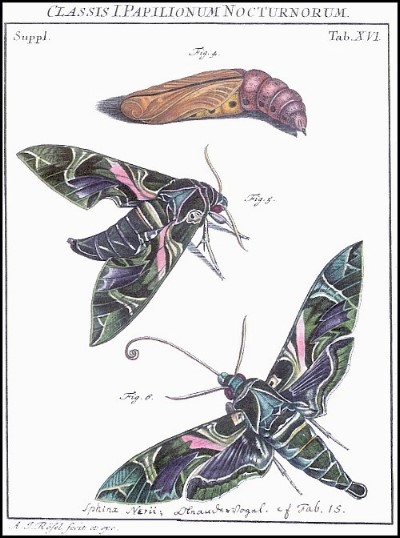
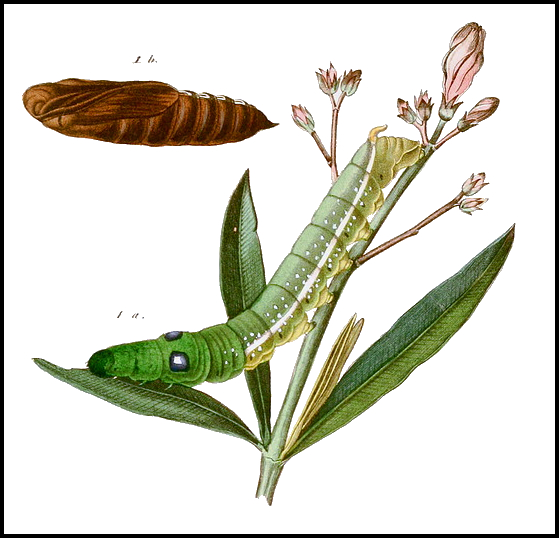
PUPA: Proboscis fused with body, a thin, black line along the former; cremaster long, slender, with a pair of apical spines; black surrounds to the spiracles.
HOSTPLANT FAMILIES: Trees and shrubs of the Rubiaceae and Apocynaceae.
UK: Oleander Hawkmoth, F: Sphinx du Laurier-Rose, D: Oleanderschwärmer, RUS: Oleandrovyi Brazhnik, S: Oleander-Svärmare, NL: Oleanderpijlstaart, CZ: Lišaj oleandrový, H: oleanderszender, E: esfinge de la adelfa, PL: Zmrocznik oleandrowiec, FIN: Oleanterikiitäjä, I: sfinge dell'oleandro, HR: oleandrov ljiljak, DK: Oleandersværmer, N: Oleandersvermer, EST: Oleandrisuru.
Sphinx nerii Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 490.Type locality: Unspecified [Europe].
(Taxonomic note. Subsp. bipartita cannot be justified as the original description was based on an abnormal specimen of Daphnis nerii.)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]
Palaeotropical.

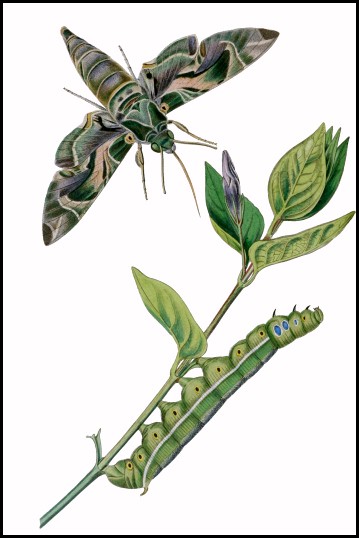
Wingspan: 90--110mm. Most easily confused with Daphnis hypothous (Cramer, 1780), a rare vagrant from India. Variation confined to the intensity of wing coloration, especially the greens and pinks; most strikingly in f. nigra Schmidt, where green is replaced by black.


A species of dry river-beds, oases and warm hillsides with scattered oleander bushes; however, localities overgrown with this shrub tend to be shunned.
Rests by day, either on a solid surface or suspended among foliage with which it blends; the head is tucked in, with the thorax and abdomen raised off the underlying substrate. Most emerge late in the evening but do not take flight until just before dawn, to feed avidly from such flowers as Nicotiana, Petunia, Lonicera, Saponaria and Mirabilis. Thereafter, flight periods are mainly just after dusk and before dawn. Under warm conditions, adults are extremely wary and, if disturbed, will take flight even during daylight hours. Pairing is a short affair usually lasting, at most, four hours but, occasionally, a couple will remain in copula until morning. Rarely found at light, unlike Daphnis hypothous (I. J. Kitching, pers. comm.).
Migrant and multivoltine in its resident range, from May to September in four or five generations, often overlapping, unlike Acherontia atropos (Linnaeus, 1758) or Agrius convolvuli (Linnaeus, 1758). In southern Europe, only two migrant-induced generations are evident -- during June and August, with individuals from the latter migrating into central and northern Europe (Lederer, 1944).
OVUM: Almost spherical, small (1.50 x 1.25mm), light green. Laid singly on both the upper and lower surfaces of young leaves of isolated, preferably sheltered, bushes, especially those at the foot of cliffs or near houses, or in clearings amongst trees. Females often fly around a plant several times before approaching with a pendulous flight. Most take up to twelve days to hatch but, during hot weather, some hatch in only five.

LARVA: Full-fed 100--130mm. Dimorphic: green or brown.





Newly-hatched larvae (3--4mm), which consume their eggshells, are bright yellow with an unusually long, very thin, blackish horn. However, with feeding the yellow quickly assumes a greenish hue and, after the first moult, the primary colour becomes apple-green with a white dorso-lateral line from abdominal segment 1 to the horn, a small, white eye-spot on the third thoracic segment, black spiracles and pink legs. With growth, the eye-spots become blue with white centres, ringed in black. The horn has an unusual bulbous 'cap' until the penultimate instar. Fully-grown larvae show little difference from younger ones, except for the change in eye-spots, and the horn losing its bulbous cap and becoming orange with a black tip, finely warted, and downward curved. In some individuals the dorsal surface is rosy, while in most the dorso-lateral line becomes edged in blue. In their final instar, some assume a bronze colour with rosy red anterior segments, which tends to mask the pre-pupation plum coloration; in all, however, the newly acquired blue-black dorsal pigmentation, the now black eye-spots and unchanged white spots on either side of the dorso-lateral line remain prominent.


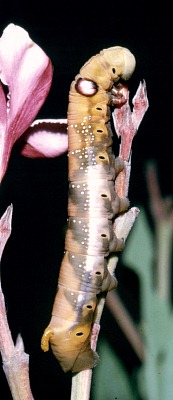
When young, larvae feed fully exposed on the topmost leaves and flowers; when larger, they tend to conceal themselves further down the branches, or even, when not feeding during daylight hours, on the ground under stones or leaf-litter. Those choosing to remain on the hostplant rest along the lower surface or stem of a leaf, with the first four segments of the body slightly hunched. When first disturbed the caterpillar stretches out to resemble an oleander leaf. With further disturbance, the anterior segments are arched up, suddenly revealing the startling eye-spots; at this point the noxious gut contents may also be regurgitated. (See also Koch & Heinig (1976).)
Found throughout its resident range between May and October; across southern Europe, sometimes in July but usually in August/September. Rarely found north of the Alps.
Major Hostplants. The flowers and young leaves of Nerium oleander.
Minor Hostplants. Vinca, Vitis, Gardenia, Alstonia, Asclepias, Jasminum, Trachelospermum, Amsonia, Carissa, Tabernaemontana, ?Mangifera, Rhazya, Adenium, Catharanthus, Ipomoea and Thevetia.
In Laos and Thailand, also recorded from Alstonia scholaris and Tabernaemontana divaricata (Eitschberger & Ihle, 2008). The latter has also been recorded from Pakistan (Muhammad Ather Rafi, pers. comm. 2014), the former from India (Dar, Jamal & Shah, 2022).
This species has been reared from ovum to imago on Lonicera caprifolium with minimal losses; however, the development time was 25% longer than on Ligustrum or Vinca and the resulting pupae were rather small, indicating a sub-optimal gustatory pathway (Mark O'Neill, pers comm 2021).
Although larvae will feed on Ligustrum ovalifolium, Lonicera caprifolium and Fraxinus excelsior in captivity, females of Daphnis nerii are reluctant to oviposit on anything other than Apocynaceae if presented with an alternative hostplant on its own or as part of a selection test. It will only take Fraxinius as a 'last resort', and will quickly switch to Ligustrum/Lonicera if it is offered (Mark O'Neill, pers comm 2021). (See also Iltschev (1919); Heinig (1976); Koch & Heinig (1976).)

PUPA: 60--75mm. Light brown, sprinkled with brown dots, particularly on wings and abdominal segments. A black line runs up the proboscis, over the head and thorax, before fading away on darker coloured abdominal segments. Cremaster short and straight. Spiracles set in a very dark brown, almost black spot. A crescent-shaped mark partly encircles each eye. Formed in a loosely spun yellow cocoon among dry debris on the ground. Rarely survives European winters, but the main overwintering stage throughout its resident range.

Braconidae: Cotesia saltator (Thunberg, 1822); Tachinidae: Drino (Palexorista) imberbis (Wiedemann, 1830).
The southern Mediterranean region, North Africa and the Middle East to Afghanistan (Ebert, 1969) and Turkmenistan (Danov & Pereladov, 1985). Along the Mediterranean, there is no clear distinction between resident and migrant populations. Permanent populations exist in suitable locations in Sicily, Crete and Cyprus (Lewandowski & Fisher, 2002); however, over a number of favourable years further colonies may be established in those islands and also in southern Italy and southern Greece, all of which die out during a hard winter.
A rare vagrant to the far north, such as to Finland, Sweden, England, Ireland (O'Connor, 1999) and the Shetland Islands (Walterson, 2003), but less rare in central Europe (Gergely, Judit & Zsolt, 2018), were it seems to be becoming more common with climate change. [However, this is not such a recent phenomenon. Selys-Longchamps (1857) noted that this species was 'common' across temperate Europe in 1835 and 1836.] In 2016 significant numbers of larvae were found in southeastern Austria (Habeler & Berg, 2016). In 2018 numerous adults and larvae were again found throughout central and western Europe, even in Spain (Monasterio León et al., 2018; Guzmán, 2018), where it rarely occurs; Ribbe (1909-1912) had noted it from Malaga and Andalucia as a rarity. In 2019 larvae of this species were common on ornamental oleanders in late summer along the Dalmatian coast of Croatia as far north as Pula (Pittaway, pers. obs. 2019).
Recorded from the Azores in the past, but now considered extinct there (Vieira, 1997).
Extra-limital range. Tropical Africa (including the Cape Verde Islands as a rare migrant (Mück & Traub, 1987)), Madagascar and the Seychelles (Fletcher, 1910; Matyot, 2005) north to the Arabian Peninsula. Also from Afghanistan eastward and southwards to South Asia (including Sri Lanka), South-east Asia, the Philippines and Palau; penetrating northwards into central southern Asia as a migrant. In 1974, this species established itself on Hawaii (Beardsley, 1979). It has also colonized the islands of Saipan and Guam (Moore & Miller, 2008), as well as Chichi-jima, Japan (Ohbayashi, 1998).
 Return to species list
Return to species list