UK: Levant Hawkmoth, F: Deiléphile Candiote; Sphinx du Levant, D: Riesenweinschwärmer; Griechischer Weinschwärmer; Orientalischer Weinschwärmer, RUS: Alekto Brazhnik, FIN: Kreetankiitäjä.
Sphinx alecto Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 492.Type locality: India.
(Taxonomic note. The coloration of adults of this species can be influenced greatly by environmental conditions experienced during the larval and pupal stages. This can give rise to environmentally produced forms, such as 'subsp.' transcaspica and 'subsp.' intermissa; neither warrants subspecific status.)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]

Palaeotropical; Oriental region. Penetrates the warmest areas of the western Palaearctic region.
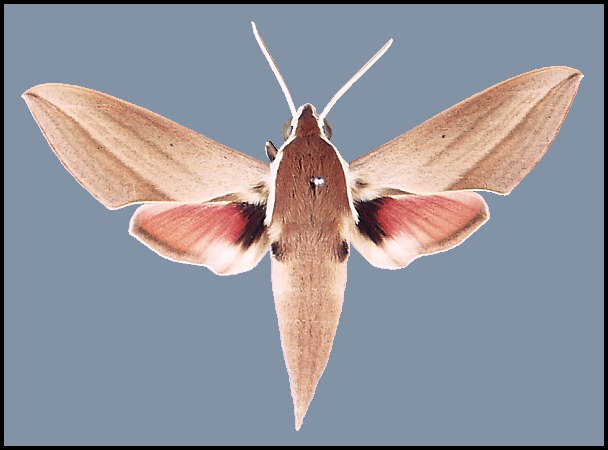
Wingspan: 80--100mm. As illustrated, with very little variation, apart from the intensity of coloration. However, a number of forms exist: f. transcaspica O. Bang-Haas bears an orange-red, oblique, submarginal line on the forewing; f. cretica Boisduval is paler than normal, with a buff tint to the forewing and orange-red hindwing. These forms are geographical, but not exclusively: the former occurs in Turkmenistan; the latter seems to be found mainly in the drier and hotter regions of south-eastern Europe and the Middle East.
In the male genitalia, uncus rather narrow, slightly concave beneath apically, clothed with erect hairs, the apex rounded, weakly sinuate. Gnathos obtusely pointed, apex upcurved, strongly convex beneath. Valve with numerous stridulatory scales. Harpe slender, weakly sigmoid, apically pointed. Phallus apically with a short, multidentate process on the right, and a long oblique row of teeth on the left, on a slightly elevated ridge that ends in a slender process lying closely on the phallus. In the female genitalia, antevaginal plate with ridge proximal to the ostium bursae rather thin, but well sclerotised and smooth, becoming lower posteriorly to form a half-moon shape.

Occurs in areas where Vitaceae are grown; in Europe, on ornamental vines rather than in vineyards due to the widespread commercial use of pesticides; in Greece, up to 1200m. The commonest sphingid in Lebanon, apart from Macroglossum stellatarum (Linnaeus, 1758) (Ellison & Wiltshire, 1939). A minor pest of grapevines in Uzbekistan (Omonov et al., 2025).
Little is known about the behaviour of this species except that it is attracted to flowers and light.


April/May, June/July and August/September, usually in three overlapping generations, with sometimes a partial emergence in October and November.
OVUM: Large (normally 2 x 1.75mm), oval, smooth, pale glossy green when first laid, but becoming yellowish after 2-3 days; not unlike the egg of Laothoe populi (Linnaeus, 1758). Up to five in a cluster may be deposited on both the upper and lower surfaces of young leaves, each female laying 150--250 eggs.
The eggs of this species vary in size, depending on the size of the female moth. Some may be as small as 1.7 x 1.45mm.

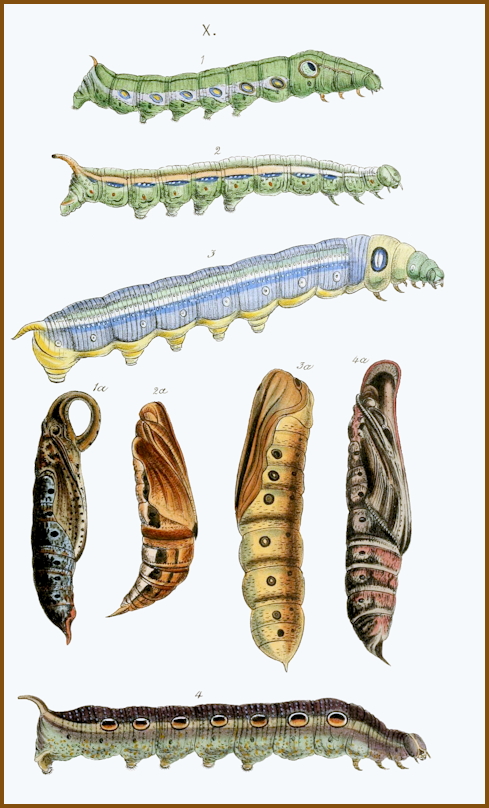
LARVA: Full-fed, 90--110mm. Trimorphic: reddish-brown, plum-brown or green.



On hatching, the 6mm-long larva is bright yellow with a very long, straight, black horn, which remains black until the final instar. With feeding, the body acquires a vine-green coloration upon which a paler, dorso-lateral line is superimposed. In the second instar, several eye-spots of diminishing size may appear along this; that on abdominal segment 1 is very bold -- black above and white below -- and, unlike some of the others, always present. Although it begins feeding from beneath a vine leaf whose colour it matches, in the fourth or fifth instar most larvae have assumed the colour and pattern of an old, gnarled vine stem, on which most now rest. Those that remain green resemble a third-instar larva of Deilephila elpenor (Linnaeus, 1758), speckled yellow, with a yellow dorso-lateral line in which, from abdominal segments 1--8, yellow-ringed eye-spots are usually present. There are also several pale yellow, oblique, lateral streaks and a blue, slightly curved, stumpy horn.
It grows very rapidly and achieves its final length and a diameter of 10--12mm in 15--25 days. With its preference for young vine shoots and its abundance in certain localities, it can cause considerable damage. Having finished feeding, it descends very quickly from the hostplant in search of a suitable place to pupate.
Most common between May and late September.
Major Hostplants. Vitis and Parthenocissus spp. In southern Turkey both Vitis vinifera and the ornamental Parthenocissus quinquefolia are taken with equal relish.
Minor Hostplants. Rubia and Gossypium, and Leea in India.
[Can be reared in captivity on lettuce (Lactuca sativa). If buying from a supermarket during the winter months, use organic produce (Bernie Betts, 2015).]
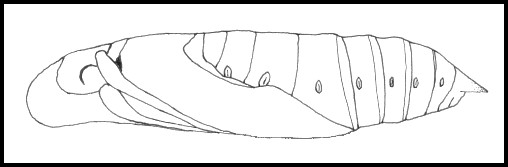
PUPA: 50--60mm. Light brown and noticeably dorso-ventrally flattened, with a granulose, keel-shaped proboscis projecting beyond the head. Cremaster short, broadly triangular, downward curving. Pupation may take place in a variety of sites: most larvae construct a loosely spun cocoon amongst dead leaves on the ground; others may pupate under stones or beneath the bark of a nearby tree, without forming a cocoon; during the summer months many pupate above ground, forming a cradle of vine leaves pulled together, usually in the notch of a stem. This stage lasts from fifteen days to five months, depending on the climate. Overwinters as a pupa.

Ichneumonidae: Hyposoter didymator (Thunberg, 1822), Mesochorus discitergus (Say, 1835) (Eisenstein, 1984); Tachinidae: Drino (Palexorista) imberbis (Wiedemann, 1830).
A species which is partially migrant, individuals having been found as far west as Sicily (Mina-Palumbo & Failla-Tedaldi, 1889; Spuler, 1908 -- although Mariani, 1939, doubts this) and north to Romania (Fleck, 1901; König, 2003); resident but rare in south-western Bulgaria (Ganev, 1984; Hristova & Beshkov, 2016) and the Republic of North Macedonia (Krpač et al., 2019). It is regularly taken in Corfu, where it is probably resident.
A rarity in Romania, with a single specimen having been collected by Lipthay, in 1934, at Baile Herculane (König, 2003).
In August 2010 larvae were found on Parthenocissus quinquefolia climbing up a church wall at Piran, Slovenia (B. Dvorak, iNaturalist 2010)
The main distribution stretches from Greece (Koutsaftikis, 1970; 1973; 1974; Fritsch et al., 2014) across southern and eastern Turkey (Daniel, 1932; de Freina, 1979; Ayberk & Akkuzu, 2005; de Freina, 2012; Akin, 2012; Koçak & Kemal, 2018; Seven, 2020; Seven & Aykal, 2022), Cyprus (Lewandowski & Fisher, 2002) to Transcaucasia (Danner, Eitschberger & Surholt, 1998), Armenia (Didmanidze, Petrov & Zolotuhin, 2013; Wąsala & Zamorski, 2015), the Republic of Georgia (Didmanidze, Petrov & Zolotuhin, 2013), Daghestan (Eberhardt, 1930), Azerbaijan (Didmanidze, Petrov & Zolotuhin, 2013; Snegovaya & Petrov, 2021) and most of Iran (Bienert, 1870; Daniel, 1961; Kalali, 1976; Ghassemi, Alemansoor & Alehossein, 2010; Lehmann & Zahiri, 2011), Turkmenistan (Danov & Pereladov, 1985; Danner, Eitschberger & Surholt, 1998), Uzbekistan (Grum-Grshimailo, 1890; Kozhanchikov, 1930; Derzhavets, 1984; Kondratiev coll., NHMUK; Shermatov et al., 2021; Shermatov & Qayumova, 2022; Omonov, Rahimov, Askarova & Khomidova, 2023; Omonov et al., 2025), Kyrgyzstan (Danner, Eitschberger & Surholt, 1998; Korb, 2018; Toropov, Milko, Zhdanko & Evdoshenko, 2023), Tajikistan, southern Kazakhstan (Titov et al., 2020; Toropov, Milko, Zhdanko & Evdoshenko, 2023) and Afghanistan (Swinhoe, 1885; Ebert, 1969; Daniel, 1971), and south to Iraq (Ramachandra Rao, 1922; Watkins & Buxton, 1923; Wiltshire, 1957), Lebanon (Lederer, 1855; Zerny, 1933; Ellison & Wiltshire, 1939), Israel (Eisenstein, 1984; Müller et al., 2005b), Palestine (Dardona, Dardona & Albayoumi, 2015), Jordan (Müller et al., 2005a; Katbeh-Bader, A., 2014), the more fertile/wetter areas of Egypt (Badr et al., 1985), and northern Libya, e.g. Susa, Benghazi (Zayeid, Aldaikh & Bomadas, 2021; Muhanad, iNaturalist 2022)).
Due to climatic changes this species has recently spread farther east in southern Kazakhstan. It has been now been found in the northern foothills of Transili Alatau Range (Almaty Region, northern part of the Tien Shan mountain massif) at altitudes ranging from 710 to 790m, and in the north-eastern foothills of Karzhantau Range (Turkestan Region, western part of the Tien Shan mountain massif) at an altitude of 550m. The records from Almaty City (Zhanna, iNaturalist 2022) are the northernmost in Central Asia and increase the known range of this species in the region by 183 km to the north-east from the previously know records from Shymkent (Titov et al., 2020).
Extra-limital range. Pakistan (Rafi et al., 2014) and India (Pathania, Sunita Sharma & Gill, 2014) to Taiwan and the Indonesian Islands.
 Return to species list
Return to species list