Type species: Sphinx populi Linnaeus, 1758.
A Palaearctic genus containing five species, of which four occur in the western part of the region.
IMAGO: Differs from Smerinthus in having a broad hindwing, sinuate between veins R1 and Sc+R1 and produced into a lobe at veins Rs and M1. Ocellus absent and anal angle strongly rounded. Male without, female with reduced frenulum; retinaculum absent. Proboscis atrophied. Male antenna less dentate than in Smerinthus ocellatus (Linnaeus, 1758), but with long setae; terminal segment short. Abdominal tergites spiny all over, the spines weak, very dense at and near the apical edges, covered with long scales. Tibiae not spinose. Foretibia without apical thorn. Hindtibia without proximal pair of spurs. Pulvillus and paronychium present.
Genitalia. In male, sacculus bipartite at apex. Phallus with spines at the edge of the sheath as well as upon the membrane of the duct. In female, lamellae without processes; the proximal edge of the ostium bursae somewhat raised, wrinkled and more strongly chitinized than rest of vaginal area.
OVUM: Almost spherical, pale green and large for the size of moth.
LARVA: Typically sphingiform; very similar to that of Smerinthus, but body stouter and horn smaller.
PUPA: Matt black or dark brown, rough (not glossy), unlike that of Smerinthus. Cremaster short, dorso-ventrally flattened, broad at base and terminating in a sharp point.
HOSTPLANT FAMILIES: Shrubs and trees, mainly Salicaceae.
UK: Poplar Hawkmoth, F: Sphinx du Peuplier, D: Pappelschwärmer, RUS: Topolevyi Brazhnik, S: Poppel-Nattsvärmare; Poppelsvärmare, NL: Populierpijlstaart, CZ: Zubokřídlec topolový; Lišaj topolový, H: nyárfaszender, E: esfinge del chopo; cuerno verde, PL: Zawisak topolowiec; Nastrosz topolowiec, FIN: Poppelikiitäjä, I: sfinge del pioppo, HR: topolin ljiljak, DK: Poppelsværmer, N: Ospesvermer, EST: Pnalaik-haavasuru.
Sphinx populi Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 489.Type locality: not stated [Sweden, Stockholm].
(Taxonomic notes. (i) In Turkey, subsp. populi and Laothoe populeti populeti (Bienert, [1870]) intergrade with each other to produce a number of intermediate forms, such as f. syriaca Gehlen, 1932a, and f. intermedia Gehlen, 1934a. Both have been synonymized with L. populeti populeti.
(ii) Under cold climatic conditions, such as in the Arctic Circle and at high altitudes in the Alps, small, dark forms sometimes occur, similar to Laothoe amurensis f. baltica Viidalepp, 1979. These are best referred to as Laothoe populi populi f. lappona (Rangnow, 1935).
(iii) Laothoe philerema (Djakonov, 1923), from Uzbekistan, Kazakhstan, Turkmenistan and Tajikistan, is now recognized as being a valid species (Daniel, 1963), as also is Laothoe austauti (Staudinger, 1877) from northwest Africa. Previously, both were considered subspecies of Laothoe populi, although Eitschberger, Danner & Surholt (1989) did consider Laothoe austauti to be a distinct species.
(iv) Laothoe populi iberica Eitschberger, Danner & Surholt, 1989 is probably not tenable: some people have even elevated this population to species level. With adult appearance and male genitalia differing little from the nominate race (superficially, individuals from southern Spain and southern France cannot be distinguished), this was established solely on a 2.7% difference in a DNA barcode sequence of one individual (Zolotuhin, 2018), with no indication given as to the natural variation in DNA barcode sequences in the nominate population across Europe.
The DNA barcodes of the holotype, allotype and two paratypes of Laothoe populi iberica have been sequenced in BOLD, although those of the holotype and one paratype are short (293bp). Nevertheless, they fall into two different BINs, with a divergence of 2.7%. The holotype and allotype, both from Andalusia in southern Spain, comprise BIN AAM9145, whereas the two paratypes, from Tereul and Segovia in central Spain, fall within a much larger cluster, BIN AAB3599, which includes all Laothoe populi samples from the UK across the Palaearctic to northern Kazakhstan. However, contrary to Zolotuhin's suggestion of sympatry, it is likely that these two clusters are allopatric, with iberica restricted to southern Spain (south of 39°N and east of 6°W) and populi occurring elsewhere on the peninsula. Regarding the DNA barcode divergence, Řezáč (2018) (STI 21910) commented in his study on Rhodoprasina that in that genus "intraspecific divergences are of the order of 4% [and] differences below 2%, unless supported by conspicuous traits in habitus and the structure of the genitalia, cannot be considered significant, even at subspecies level". This seems to be generally true of many Smerinthini that do not feed as adults and thus, in the absence of such morphological differences, the divergence of 2.7% between iberica and populi indicates subspecies differentiation at best (Ian Kitching, pers. comm.). Taken together, there is thus no justification for treating Laothoe iberica as a species separate from Laothoe populi but rather as a subspecies, namely Laothoe populi iberica, but even this is probably not tenable. Traditional hybridization experiments between the two populations may settle this issue, as they have done for Laothoe austauti (Staudinger, 1877) and Laothoe populi populi (Linnaeus, 1758). However, the relationship of this southern population to Laothoe austauti (Staudinger, 1877) still requires further study.
(v) The specimen illustrated by Danner, Eitschberger & Surholt (1998) from the Taibai Shan, central China, has recently been described as a new species, namely Laothoe habeli Saldaitis, Ivinskis & Borth, 2010.)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]

Holarctic; western Palaearctic region. Pleistocene refuge: Monocentric -- Pontomediterranean refuge.
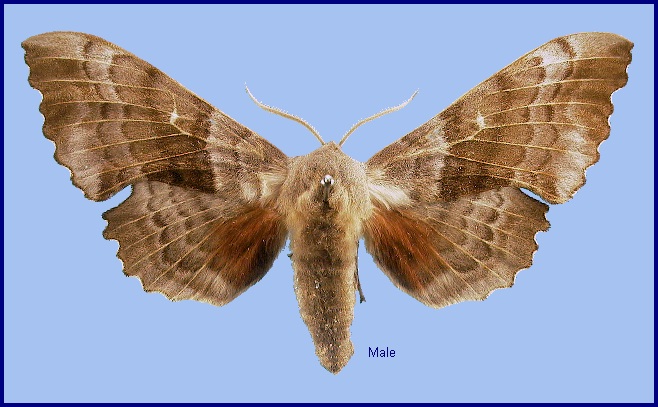
Wingspan: 70--100mm. A very distinct species with rust-red hindwing patches, confusable only with Laothoe austauti (Staudinger, 1877), Laothoe populeti (Bienert, [1870]), Laothoe philerema (Djakonov, 1923) and Laothoe amurensis (Staudinger, 1892). Ground colour of the wings varies considerably from pale buff, through pale browns, reds (mainly in females), and greys to an almost black suffusion. Likewise, the transverse lines can be either very prominent, or totally absent, while the hindwing patches may be any shade of red or brown, and any size. Gynandromorphs are common, and if each half is a different colour, the effect can be striking. Developing pupae subjected to heat produce pale buff to rose-red adults. Cold conditions can produce adults which are dark grey.







The region's commonest hawkmoth, and one of the most widespread. Frequents almost any damp, low-lying area, such as country lanes, open woodland rides, railway cuttings or town parks, particularly where Populus spp. but also Salix spp. are present; commoner where the former occurs. Up to 1600m in the Alps. Most emerge late at night or early in the morning, clambering up the tree trunk at the base of which the larva had pupated. Not until the following evening does the moth take flight, females quickly selecting a resting position amongst foliage from which the males are attracted at around midnight. Once paired, they remain coupled until the following evening when, after separation, the females start laying eggs almost immediately.
Like with Smerinthus ocellatus (Linnaeus, 1758), numbers may fluctuate from year to year due to the attentions of larval parasitoids, especially Microplitis ocellatae Bouché, 1834. A description of the life history of this parasitic wasp, and its effects on the host, are outlined by Ellis (1996).

At rest, the hindwings protrude considerably beyond the forewings, so that amongst foliage this attitude, combined with their colour and cryptic pattern, creates a very good resemblance to a cluster of dead poplar leaves. However, individuals are attracted to light in large numbers and are then very conspicuous.
Generally univoltine over its northern range, from mid-May to mid-July, with a peak towards mid-June in most years. However, due to the very warm weather experienced in southern England during 1990, this species peaked a month earlier and produced a second generation in August. Over southern Europe and Asia Minor, usually bivoltine in May and early June, and again in August, although in warm years and certain hot localities, adults may be found in April/May, July and early September.
OVUM: Large (1.7 x 1.5mm), almost spherical, pale green, glossy. Between 57 and 171 can be laid per female (Williams, 1966), with these being attached, singly or in pairs, to the undersurface of leaves less than 2m from the ground. However, on the large hybrid black poplar (Populus x euramericana) and tree willows lacking side shoots or suckers, eggs are laid high in the crown. Depending on temperature, they take 11--17 days to hatch (Williams, 1966).



LARVA: 65--85mm. Trimorphic: green, whitish grey and bluish white.



The newly-emerged 5mm-long larva, which partially devours its eggshell, is pale green, very rough, with small, yellow tubercles, and has a rounded head and a cream-coloured horn. With growth, yellow lateral stripes appear and the legs and spiracles become pink; the body colour, however, usually remains yellowish-green, with yellow tubercles. Occasionally, some individuals become heavily spotted with red, especially those feeding on Salix as opposed to Populus spp. Others may be bluish-white with cream stripes and tubercles, or even nearly white, instead of the basic green and yellow coloration, especially those feeding on Populus alba (see Grayson & Edmunds, 1989b, for an explanation of this). A few totally red individuals are also known (Jean Haxaire, pers. obs. 2018). When full-grown, all are very stocky, whatever the colour form, and develop a brownish tint prior to pupation.







Initially, the young larva rests along a vein on the underside of a leaf but, after the second moult, it adopts a more characteristic sphinx-like posture, hanging beneath a leaf or a stalk by its last two pairs of prolegs (Williams, 1966). Not very active, it tends to remain in the same feeding area throughout its life, noticeably stripping numbers of shoots.
In northern Europe, occurs from June to early September; farther south, individuals at all stages of development can be present at any time between May and late September. An individual from Oktyabr'skoe (western Siberia, 62°30'N) was captured on 22.vi.1963 (Vladimir Dubatolov, pers. comm. 2012).
Major Hostplants. Many species and cultivars of Populus and Salix.
Minor Hostplants. Recorded from Fraxinus, Quercus, Betula, Alnus, Rosa, Crataegus, Cotoneaster, Malus, Laurus and, in Spain, Ulmus. It should be noted, however, that the ability of populations to feed on these secondary hostplants must be a localized phenomenon. Larvae from Oxfordshire, England, and/or Czechia, refused Betula, Quercus cerris, Viburnum tinus, Fraxinus or Ulmus, resulting in 100 per cent mortality; all larvae of the same broods which were offered Populus completed their development (Pittaway, 1996). Records of Viburnum tinus as a hostplant (Lucas, 1895; Barrett, 1895; Tutt, 1902; Newman, 1965; Pittaway, 1993) must be erroneous; no larvae have ever been confirmed as feeding on this plant and all attempts to entice larvae from England and Czechia to feed (as well as larvae of Laothoe populeti populeti (Bienert, [1870]) from Hakkari, Turkey), have failed (Pittaway, 1996).
PUPA: 30--43mm. Usually matt black, but sometimes reddish-brown, rough and stout, blunt anteriorly. Cremaster triangular, terminating in a sharp, glossy spine beneath which two rough projections are present. Initially, completely covered with a fine dark brown 'skin' which peels off in strips. Formed in an earthen cell 2--3cm below soil level (or under damp leaves) at the base of its hostplant. The overwintering stage.

Ichneumonidae: Amblyjoppa proteus (Christ, 1791), Amblyteles armatorius (Förster, 1771), Aphanistes ruficornis (Gravenhorst, 1829), Banchus pictus Fabricius, 1798, Callajoppa cirrogaster (Schrank, 1781), Coelichneumon deliratorius (Linnaeus, 1758), Diphyus monitorius (Panzer, 1801), Heteropelma capitatum (Desvignes, 1856), Hoplocryptus murarius (Borner, 1782) [syn. Aritranis fugitivus (Gravenhorst, 1829)], Microleptes splendidulus Gravenhorst, 1829, Netelia testacea (Gravenhorst, 1829), Netelia vinulae (Scopoli, 1763), Pimpla illecebrator (Villers, 1789), Protichneumon pisorius (Linnaeus, 1758); Braconidae: Cotesia glomerata (Linnaeus, 1758), Microplitis ocellatae Bouché, 1834, Microplitis tuberculifer (Wesmael, 1837), Microplitis vidua (Ruthe, 1860), Aleiodes praetor (Reinhard, 1863) [syn. Rogas praetor Reinhard, 1863]; Eulophidae: Eulophus smerinthicida Bouček, 1959; Encyrtidae: Ooencyrtus telenomicida (Vassiljev, 1904), Ooencyrtus vinulae (Masi, 1909); Trichogrammatidae: Trichogramma evanescens Westwood, 1833; Scelionidae: Telenomus punctatissimus (Ratzeburg, 1844); Tachinidae: Blondelia nigripes (Fallén, 1810), Compsilura concinnata (Meigen, 1824), Drino lota (Meigen, 1824), Exorista sorbillans (Wiedemann, 1830), Exorista xanthaspis (Wiedemann, 1830), Frontina laeta (Meigen, 1824), Masicera sphingivora (Robineau-Desvoidy, 1830), Oswaldia spectabilis (Meigen, 1824), Pales pavida (Meigen, 1824), Thelaira nigripes (Fabricius, 1794), Winthemia bohemanni (Zetterstedt, 1844), Winthemia cruentata (Rondani, 1859), Winthemia quadripustulata (Fabricius, 1794), Winthemia rufiventris (Macquart, 1849).
Cordiceps militaris (Linnaeus) has been recorded as a larval pathogen in Sweden (Lagebberg, 1922). The Encyrtidae, Ooencyrtus vinulae, was reared from an egg collected in London, UK, on 27.vii.2012 (John Noyes/David Notton, pers. comm. 2012).


From the Western Isles of Scotland (Heslop Harrison, 1958), Ireland (Lavery, 1991), Portugal (Corley, 2004; Pires & Corley, 2007; Marabuto, 2018) and Galicia, Spain (Pino Pérez et al., 2009; Hiernaux, Hurtado & Fernández, 2010; Fernández Vidal, 2016) eastwards across the Urals (Eversmann, 1844; Pittaway, 1983b; Derzhavets, 1984) to western Siberia and the Altai (Zolotarenko, Petrova & Shiryaev, 1978; Izerskiy, 1999), as well as northwestern China (Xinjiang Province) south to Tacheng (Pittaway & Kitching, 2000). Also the Ukraine (Khalaim, 2022), southern Russia, and western/northern Turkey (Daniel, 1939; de Freina, 1979; Ayberk & Akkuzu, 2005; de Freina, 2012; Okyar, 2012). It has also been confirmed from Sicily (Mina-Palumbo & Failla-Tedaldi, 1889; Parenzan, 1995; Parenzan & Porcelli, 2006; Bella, Parenzan & Russo, 2010), Corsica (Julien Bottinelli, iNaturalist 2017) and Sardinia (Parenzan, 1995), as well as northern and central Greece, including the island of Samos (Fritsch, Stangelmeier, Top-Jensen & Bech, 2014). Hariri (1971) recorded this subspecies from western Syria; this has been confirmed by Talhouk (1997). This population is probably contiguous with that found in Adana Province, Turkey (Feza Doganlar, pers. comm.).
Recorded as far north as Arkhangelsk Oblast (Kozlov, Kullberg & Zverev, 2014) and Ukhta (Tatarinov, Sedykh & Dolgin, 2003) in european Russia, and Oktyabr'skoe in western Siberia (Ob' River, 62°30'N) (Vladimir Dubatolov, pers. comm 2012).
The poorly differentiated semi-isolated population in southern and eastern Spain, described as Laothoe populi iberica Eitschberger, Danner & Surholt, 1989, occurs in the provinces of Jaén (Lara Ruiz, 2011), Granada (Lara Ruiz, 2011), Almeria (Garre, Rubio, Guerrero & Ortiz, 2020), Cuenca and Teruel. Rambur (1942) recorded it as being common along rivers around Malaga, and Aistleitner & Aistleitner (1998) recorded it from Albacete (Murcia).
In northwestern China the Dzungarian Gate/Gap and the inhospitable deserts to either side appear to separate the more northern Laothoe populi populi from the semi-isolated southern population of Laothoe populi populetorum (Staudinger, 1887), although this narrow barrier may be breaking down due to the extensive planting of poplar trees. Three other species have reached the Yining/Gulja area by exploiting planted trees, namely Smerinthus ocellatus, Smerinthus planus and Callambulyx tatarinovii tatarinovii (Pittaway & Kitching, 2000).
Absent from the Caucasus region and Transcaucasia, where it is replaced by the closely related Laothoe populeti populeti (Bienert, [1870]) and Laothoe populeti caucao Zolotuhin, 2018 (Didmanidze, Petrov & Zolotuhin, 2013; Zolotuhin, 2018; Streltzov et al., 2022).
Extra-limital range. Siberia as far east as Irkutsk (E. Berlov, pers. comm.; Natalya Fomina, iNaturalist 2021) and the Barguzin/Ulan-Burgasy Mountains (Transbaikalia, east bank of Lake Baikal) (V. Dubatolov, pers. comm. 2010; Gordeev & Gordeeva, 2021). It is only in recent years that this species has been recorded from southern Siberia, first from Novosibirsk and Tomsk (Izerskiy, 1999), then Irkutsk, then Dobo-Yonkhor, Transbaikalia. It was considered common in 2012-2019 at Reka Khara-Atsagat (Gordeev & Gordeeva, 2017). It is possible that Laothoe populi populi is extending its range eastwards.
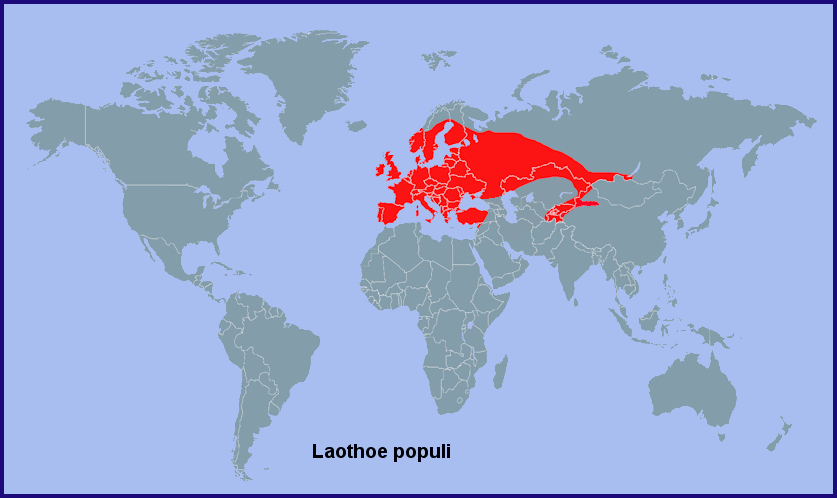
River valleys in the mountains and foothills of eastern Uzbekistan, Tajikistan, Kyrgyzstan and south-eastern Kazakhstan (south of the Kyrgyz Steppe) to northwestern China as subsp. Laothoe populi populetorum (Staudinger, 1887).
 Return to species list
Return to species list