Type species: Sphinx elpenor Linnaeus, 1758.
A Palaearctic genus containing four species.
IMAGO: Labial palpus rough, with long lateral hairs; scaling on inner surface as in Hyles. Eyelashes much more distinct than in Hyles. Female antenna clubbed, male almost filiform; terminal segment short. Spines on abdomen numerous, less strongly chitinized than in Hyles. First row of spines of the first protarsal segment doubled at base. Pulvillus always present.
Genitalia. Uncus slender, much narrower than gnathos; the latter flat, or slightly convex beneath, not keeled or boat-shaped, rounded-truncate or rounded at end. Valva broadly sole-shaped, with a dozen or more friction scales. Sacculus ending in a more or less spatulate process, which is concave on upperside and slightly curved upwards. Phallus without apical process, but with a strong, subapical, oblique, dentate ridge.
OVUM: Ovoid, pale glossy green.
LARVA: Not typically sphingiform: anal horn reduced or absent; thoracic segments and head retractile into the eye-spot-bearing first and second abdominal segments. Oblique lateral bars present.
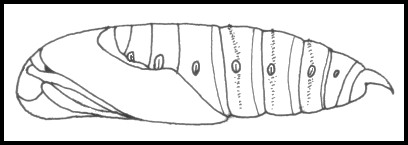
PUPA: Proboscis fused with body, weakly carinate. Similar to those of Hippotion and Theretra but with a dorso-lateral transverse row of fine, caudad-pointing hooks on each motile abdominal segment. Cremaster broadly triangular and down-curving, without terminal spines.
HOSTPLANT FAMILIES: Herbaceous plants, mainly of the Onagraceae and Rubiaceae.
UK: Large Elephant Hawkmoth, F: Sphinx de la Vigne, D: Mittlerer Weinschwärmer; Schotenweiderich Schwärmer, RUS: Srednii vinnyi Brazhnik, S: Allmän Snabelsvärmare; Större snabelsvärmare, NL: Groot Avondrood, CZ: Lišaj vrbkový, H: szőlőszender, E: esfinge de la vid, PL: Zmrocznik gladysz, FIN: Horsmakiitäjä, I: sfinge della vite, HR: veliki vinski ljiljak, DK: Dueurtsværmer, N: Stor snabelsvermer, EST: Suur-punasuru.
Sphinx elpenor Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 491.Type locality: Unspecified [Europe].
(Taxonomic note. According to I. J. Kitching (pers. comm.), the characters which separate Deilephila elpenor lewisii (Butler, 1875) from Deilephila elpenor elpenor are not confined to that subspecies. Individuals of what are typical subsp. lewisii can be found within the range of subsp. elpenor; the reverse is also true. Subsp. lewisii was therefore synonymized with subsp. elpenor by Pittaway (1993).)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]


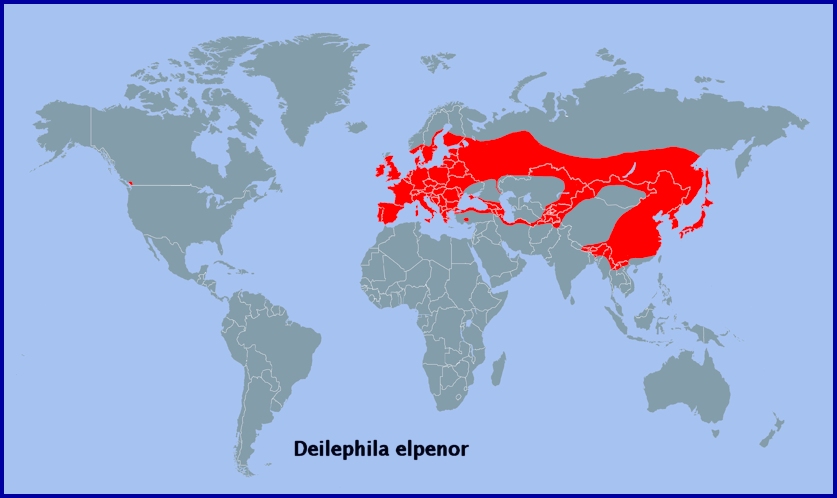
Holarctic; Palaearctic (both eastern and western subregions). Pleistocene refuge: Polycentric -- Holomediterranean, Caspian, Iranian, Turkestan and Manchurian refugia.

Wingspan: 60--75mm. A very distinct species and difficult to confuse with any other in the region except Deilephila rivularis (Boisduval, 1875). Upperside of head, thorax and abdomen khaki, except for the inner edges and median line of the tegulae, posterior margin of thorax, base of abdomen, abdominal median line and terminal abdominal segments, all of which are pink. Upperside of forewing khaki, except for costa, a narrow, median band extending from the inner margin to M3, a narrow postmedian band extending from the inner margin to the apex, and the marginal band, all of which are pink. Basal half of hindwing upperside black, distal half pink; distal edge of black area almost straight and parallel to outer margin, so that pink area is of almost even width across the wing. Variation is confined to the extent of or, as in the case of f. unicolor Tutt, absence of pink coloration on the forewing.
Male genitalia similar to those of Deilephila porcellus (Linnaeus, 1758) but uncus more slender. Gnathos more rounded distally. Stridulatory scales of valve more numerous. Harpe longer. Apical dentate process of phallus longer.


Frequents the flood-plains of streams and rivers, but also occurs in damp forest clearings, town wastelands or railway cuttings; up to 1500m in the Alps.



Hiding away amongst foliage by day, adults do not take flight, unlike many other hawkmoths, until well after dark, when scented flowers such as Lonicera, Silene, Buddleja and Valeriana are avidly visited: scent is the main source of attraction in this species (Balkenius, Rosén & Kelber, 2006). Towards midnight, pairing takes place, with pairs rarely remaining in copula for more than two hours. Following separation, females immediately begin to lay eggs, continuing to do so over a number of nights until approximately 100 have been deposited. It is strongly attracted to light, with large numbers often coming to mercury-vapour lamps between 22.00 and 24.00 hours.

Late May to early July, with a partial second generation in southern Europe from mid- to late August. In exceptional years, some adults may also be found in August in southern England (Tutt, 1904). In the southern Urals, from late May until early July (Nupponen & Fibiger, 2002); in southern Portugal, mainly in April and May (Corley, 2004).
OVUM: Almost spherical (c. 1.5 x 1.2mm), pale glossy green. Laid singly or in pairs underneath the leaves of its hostplant, hatching about ten days later.
LARVA: Full-fed, 70--85mm+. Dimorphic: brown or green.





Newly-hatched larva (4--5mm long) pale green, cylindrical, with a small, narrow horn. In the second instar the head is disproportionately small. With a further moult, the first and second abdominal segments enlarge and have large and extremely realistic eye-spots. It is during this instar that most change to the final dark form, although some remain green or turn into a rare blue-grey form (Pascal Régnier, pers. comm. 2014). In between feeding, both by day and at night, the young larva rests stretched out beneath a leaf, where it is extremely well camouflaged. Later, larger individuals feed fully exposed at the top of a plant by day (Theunert, 1990) or night, preferring flowers and seed-heads to leaves. When not feeding, it often retires to hide at the base of the plant where its dark coloration is of greater advantage. Some larvae, especially those on Galium, may feed openly only at night, often in the company of those of Macroglossum stellatarum (Linnaeus, 1758) and Deilephila porcellus.
Larvae are regularly found on emergent aquatic hostplants in northern Europe, such as Menyanthes trifoliata (Bog Bean) and Zantedeschia aethiopica (Arum Lily). The larva can 'swim' if it drops from these plants into the water below, and may even crawl through the water to reach other plants or dry land.
A striking feature of this species is its defensive behaviour. When alarmed, the head and the three thoracic segments are withdrawn into the first and second abdominal segments, which expand greatly, enlarging the startling eye-spots. Even quite large birds have been known to flee at this sight. The coloration of these 'eyes' remains bright until pupation.
Occurs between early June and late September, in overlapping generations.
Major Hostplants. Epilobium and, to a lesser extent, Galium.
Minor Hostplants. Fuchsia, Impatiens, Lythrum, Calla, Zantedeschia, Menyanthes (Schroeder, 1990) and Lonicera in northern Europe. In its southern range also on Vitis (Kozhanchikov, 1930; Rambur, 1942), Parthenocissus, Circaea, Oenothera, Arisaema, Polygonum, Daucus, Lysimachia and Rumex. It used to be an occasional major pest of grapevines in southern Europe (Dekhtyarev, 1929), but rarely so today.
PUPA: 35--47mm. Similar to those of Hippotion celerio (Linnaeus, 1758) and Theretra alecto (Linnaeus, 1758), but easily distinguished by having a strongly reduced proboscis; brown coloration streaked with even darker brown; and a single row of spines on each mobile, abdominal segment. The function of these has yet to be ascertained, but Brock (1990) has demonstrated that if cocoons of this species are flooded or exposed to high humidity, up to 90% of pupae rapidly work their way out of the cocoon by using these spines. This behaviour may be an adaptation to avoid drowning during flooding of the habitat. Even so, pupae are very active, often working their way out of their cocoon prior to emergence. Pupation takes place very close to the hostplant in a strongish mesh cocoon amongst debris on the ground. Overwinters as a pupa.



Ichneumonidae: Amblyjoppa fuscipennis (Wesmael, 1845), Amblyjoppa proteus (Christ, 1791), Barylypa propugnator (Förster, 1855), Cratichneumon sicarius (Gravenhorst, 1829), Dusona cultrator (Gravenhorst, 1829), Enicospilus undulatus (Gravenhorst, 1829), Pimpla illecebrator (Villers, 1789), Pimpla rufipes (Miller, 1759), Protichneumon pisorius (Linnaeus, 1758), Protichneumon similatorius (Fabricius, 1798), Pyramidophorus flavoguttatus Tischbein, 1882, Therion circumflexum (Linnaeus, 1758); Tachinidae: Blepharipa pratensis (Meigen, 1824), Compsilura concinnata (Meigen, 1824), Cyrtophloeba ruricola (Meigen, 1824), Drino (Zygobothria) atropivora (Robineau-Desvoidy, 1830), Drino lota (Meigen, 1824), Drino vicina (Zetterstedt, 1849), Exorista grandis (Zetterstedt, 1844), Exorista larvarum (Linnaeus, 1758), Hubneria affinis (Fallén, 1810), Isosturmia picta (Baranov, 1932), Masicera sphingivora (Robineau-Desvoidy, 1830), Oswaldia spectabilis (Meigen, 1824), Pales pavida (Meigen, 1824), Tachina lurida (Fabricius, 1781), Thelaira nigripes (Fabricius, 1794), Winthemia cruentata (Rondani, 1859), Winthemia quadripustulata (Fabricius, 1794).

Sphingidae are the chief hosts of Drino lota, with brood sizes of up to 27 known.

In Britain, the larvae of Deilephila elpenor elpenor are parasitized by Amblyjoppa proteus (Christ) (Ichneumonidae), a fact which was recorded by Albin (1720), Dutfield (1748), Wilkes (1749) and Harris (1766); the adult ichneumon is easily recognized from their excellent colour plates.
A semi-migrant; resident throughout temperate Europe (Newman, 1965), including Sicily (Mariani, 1939; Parenzan & Porcelli, 2006; Bella, Parenzan & Russo, 2010), Salento in southern Italy (Durante & Pellegrino, 2019), Corsica, Sardinia (Parenzan & Porcelli, 2006), the Republic of North Macedonia (Krpač et al., 2019), Greece (including Corfu) and Bulgaria (Karisch, 1990), across to western Siberia and the Altai Mountains (Zolotarenko, Petrova & Shiryaev, 1978; Izerskiy, 1999). Also Turkey (de Freina, 1979; Koçak & Kemal, 2018), Moldova (Tugulea & Tugulea, 2020), Ukraine/Crimea (Dekhtyarev, 1929; Khalaim, 2022) and lower Volga river (Zolotuhin, pers. comm.), the Alborz Mountains of northern Iran (Watkins & Buxton, 1923; Sutton, 1963; Barou, 1967; Ebert, 1976; Lehmann & Zahiri, 2011), and the Republic of Georgia (Didmanidze, Petrov & Zolotuhin, 2013; Streltzov et al., 2022), Armenia (Didmanidze, Petrov & Zolotuhin, 2013) and Azerbaijan (Didmanidze, Petrov & Zolotuhin, 2013; Snegovaya & Petrov, 2021). It is also present in eastern Kazakhstan (Alpheraky, 1882; Danner, Eitschberger & Surholt, 1998; Toropov, Milko, Zhdanko & Evdoshenko, 2023), Kyrgyzstan (Korb, 2018; Toropov, Milko, Zhdanko & Evdoshenko, 2023), and northern and southeastern Uzbekistan (Kozhanchikov, 1930; Toropov, Milko, Zhdanko & Evdoshenko, 2023; Omonov et al., 2025) north to the Altai Mountains (Knyazev, 2023). Absent from northern Scandinavia, northern Russia, most of the Middle East, and North Africa (Rungs, 1981), but recorded as far north as Arkhangelsk Oblast (Kozlov, Kullberg & Zverev, 2014) and Ukhta (Tatarinov, Sedykh & Dolgin, 2003) in european Russia.
On the Iberian Peninsula, this species is found in northern and eastern Spain as far west as Galicia (Pino Pérez et al., 2009; Fernández Vidal, 2016) and as far south as Malaga (Ribbe, 1909-1912), and down to southern Portugal (Corley, 2004; Pires & Corley, 2007). There is one record from the Balearic Islands (Pérez De-Gregorio et al., 2001).
This species was recorded from north-eastern Afghanistan by Ebert (1969), but in his 1974 publication he points out that this was an error and that the specimen in question was Deilephila rivularis (Boisduval, [1875]).
Deilephila elpenor has also been reported from northern Pakistan (Humairah Hanif et al., 2016). However, the specimens illustrated match the pale form of Deilephila rivularis. Interestingly, Sphinx ligustri was also reported from the same area by the same authors. Both species may have only recently spread south into this area as Bell & Scott (1937) failed to find either, but this requires confirmation. The latter, however, has also been reported from northwestern India (Smetacek, 1994).
Extra-limital range. From western Siberia (Eversmann, 1844; Zolotarenko, Petrova & Shiryaev, 1978) across southern Siberia and southern Yakutia/Sakha (Kaimuk et al., 2005) to the Russian Far East (Derzhavets, 1984; Izerskiy, 1999), Sakhalin Island (Dubatolov, 1991), the Kurile Islands (Dubatolov, 1991), Korea (Danner, Eitschberger & Surholt, 1998) and Japan (Inoue et al., 1982). Also northern and central China as far south as the Tian Shan, Xizang Province/Tibet, Sichuan Province and Nanjing (Chu & Wang, 1980b).
Also southern British Columbia (Canada) and northern Washington State (USA), as a recent introduction.
Nepal, Sikkim, Bhutan, Assam (India), northern Myanmar and Yunnan Province, China, as subsp. macromera (Butler, 1875).
Subsp. szechuana (Chu & Wang, 1980a) from Sichuan Province, China, is not valid as it was described from a mouldy specimen, the fungus having dulled the colours (Pittaway & Kitching, 2000). Fresh specimens from Sichuan are indistinguishable from subsp. elpenor.
 Return to species list
Return to species list