Type species: Sphinx tiliae Linnaeus, 1758.
A Palaearctic genus with two species.
IMAGO: Very similar to Marumba, but forewing lobate distally. Proboscis very short and atrophied. Labial palpus with rough scaling and hair, the joint between segments 1 and 2 covered by the scaling and not visible from the outside as a naked spot; much smaller in female than male. Antenna triangular in cross-section in male, with lateral, seta-bordered grooves, the upper edge of which is laterally produced; cylindrical in female, without lateral grooves and prolonged setae. Apical segment short in both sexes. Abdominal spines restricted to segment edges, soft, sparse and partly modified into scales; ventral scales long. Tibiae with spurs; foretibia without apical thorn, epiphysis long, almost extending to apex of tibia; hindtibia with two pairs of spurs. Pulvillus, paronychium, retinaculum and frenulum present. Vein M3 of hindwing before centre of cell.
Genitalia. In the male, valva rounded or subtriangular. Sacculus separated distally by a shallow, sinuate incision to give two small tooth-like apical lobes, which are occasionally rough at edges with small notches. Phallus with a movable apical process, which is armed apically with two small sharp cornuti, these varying in size and position. In the female, lamellae rather large; proximal edge of ostium bursae raised, shallowly sinuate.
OVUM: Ovoid, pale green, with a brown tint developing after a few days.
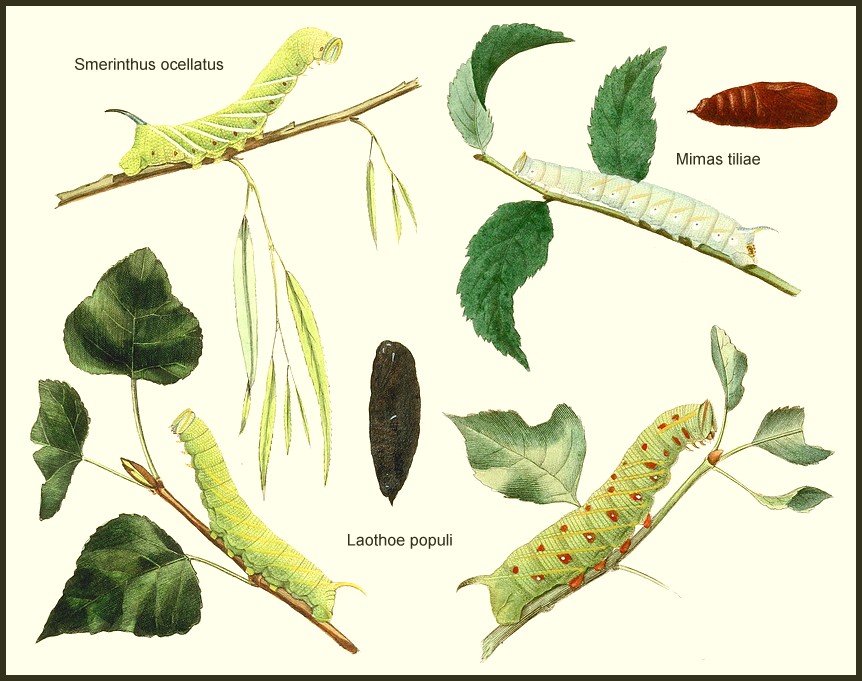
LARVA: Resembles that of Marumba, but tapers anteriorly when fully grown, with a distinct, yellow, tuberculate structure on anal flap. Horn erect, slightly curved and granulose.
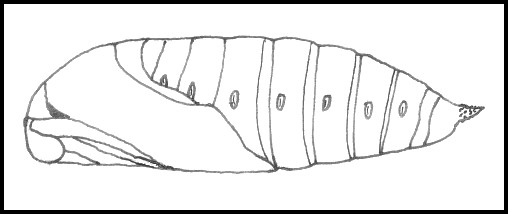
PUPA: Similar to that of Marumba and Smerinthus but, like the latter, lacks frontal ridges; also rough instead of glossy; more elongate. Cremaster curved dorsally, with a pair of spines on an extended tip and a few lateral ones.
HOSTPLANT FAMILIES: Mainly trees of the Tiliaceae, Fagaceae, Betulaceae, Ulmaceae and Rosaceae.
UK: Lime Hawkmoth; Linden Hawkmoth, F: Sphinx du Tilleul, D: Lindenschwärmer, RUS: Lipovyi Brazhnik, S: Lind-Nattsvärmare; Lindsvärmare, NL: Lindepijlstaart, CZ: Lišaj lípový, H: hársfaszender, E: esfinge del tilo, PL: Zmrocznik lipowiec; Nastrosz lipowiec, FIN: Lehmuskiitäjä, I: sfinge del tiglio, HR: lipin ljiljak, DK: Lindesværmer, N: Lindesvermer, EST: Pärnasuru.
Sphinx tiliae Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 489.Type locality: Unspecified [Europe].
(Taxonomic notes. (i) At high altitudes, or when pupae are chilled just prior to the emergence of the moth, a darker form, f. montana Daniel & Wolfsberger, 1955, is produced, which has been regarded as a subspecies by some, but this is unwarranted.
(ii) Genetic evidence suggests that one or more of the isolated populations from Turkey, Transcaucasia, Daghestan and Azerbaijan may warrant subspecific status (Ian Kitching, pers. comm. 2011). In the adults, the wings are more pointed, and reddish-brown in both sexes [see below for photo]. However, a DNA barcode sequence of a single individual from Azerbaijan places this population firmly within the nominate subspecies (Ian Kitching, pers. comm 2015). This requires further study.
(iii) DNA barcode analysis indicates that the main European population is composed of two refugial entities, one which survived the last ice age in the Iberian Peninsula, the other in the southern Balkans. Since the end of the ice age these have spread north to the west and east of the Alps, respectively; they now overlap in modern day Germany. It is not known for sure whether these two entities have retained their separate identities (making them good (cryptic) species), or whether they are now interbreeding (most likely). The eastern european population has been given the name Mimas tiliae orientalis Melichar, Melichar, & Řezáč (2021), but this situation requires further study via traditional hybridization experiments.
(iv) The semi-isolated population from the Alborz mountains of northern Iran was described as a separate species in 2015, namely Mimas kitchingi Melichar & Řezáč, 2015. With adult appearance and male genitalia differing little from the nominate race, this was established solely on a 2.73% difference in a DNA barcode sequence between one individual from both the European and Alborz populations. With no indication given as to the natural variation in DNA barcode sequences in the nominate population of Europe [the largest difference in BOLD, between two specimens of Mimas tiliae tiliae from the UK and Turkey, is about 2.96% (Ian Kitching, pers. comm. 2015)], the elevation of the Iranian population to species level is somewhat premature. Only traditional hybridization experiments between the two populations will settle this issue, as they have done for Laothoe austauti (Staudinger, 1877) and Laothoe populi populi (Linnaeus, 1758).)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]
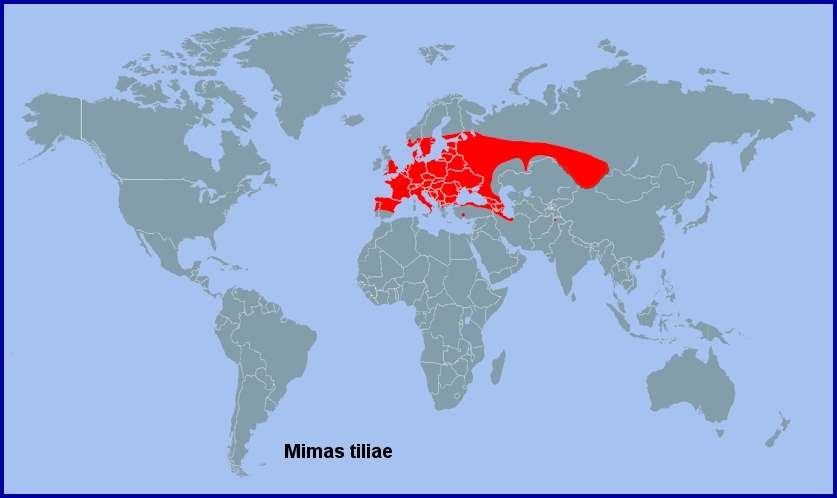
Holarctic; western Palaearctic region. Pleistocene refuge: Polycentric -- Holomediterranean refugia.
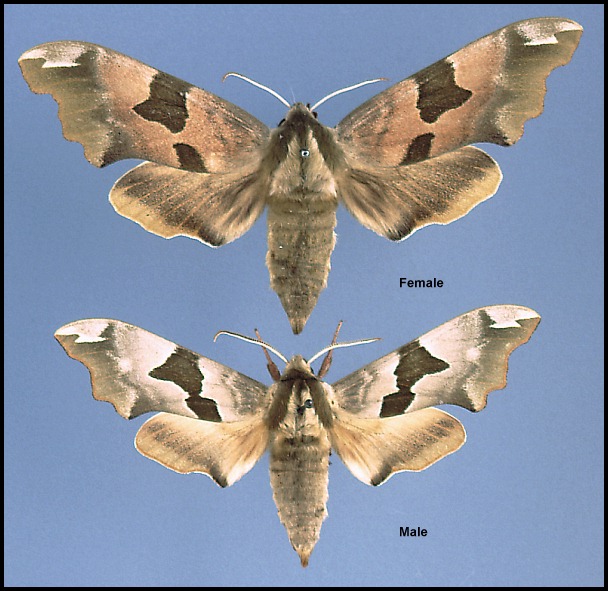


Wingspan: 60--80mm. Sexually dimorphic; a very distinct species, not confusable with any other sphingid of the area despite being highly variable. Ground colour brown (f. brunnea Bartel), grey (f. pallida Tutt), yellow (f. lutescens Tutt) or green (f. virescens Tutt). Sexual dimorphism marked: normally, the forewing ground colour is brownish in females and decidedly green in males, but there are many exceptions. The wing pattern is also very variable, the dark median band of the forewing being entire (f. transversa Tutt), narrowly interrupted (f. tiliae), or completely absent (f. obsoleta Clark). Gynandromorphs are also quite common. Sexual dimorphism is also evident in body-shape: the female abdomen is straight and fat because most eggs are already fully formed when the female emerges, as in all species of Smerinthini. The male abdomen, on the other hand, is strongly curved and slender.






Rarely found outside deciduous woodland areas, where a preference is shown for open river-valley vegetation, rich in Tilia and Ulmus species; avenues of trees lining suburban and urban roads and parks; plantations and orchards of cherries (especially in central Europe); and warm, wet mountain sides overgrown with Alnus viridis. Up to 1500m in the Alps.
After emergence during the morning, many adults are to be found resting on the trunks of host-trees, where they have expanded their wings. Subsequently, most prefer to hang suspended amongst foliage. It is here that mating pairs are to be found -- the male hanging below the female and remaining in this position for up to 20 hours before dropping to the ground and flying off at dusk. The female takes flight shortly after dusk and almost immediately commences egg-laying. However, they are active for only a short period and two hours after sunset few adults are still flying. As Mimas tiliae tiliae does not feed, the adults are not attracted to flowers, but large numbers of males come to light or a virgin female.
Izerskiy (1999) states that in western Siberia females of this species are generally active from 22.50h until 01.40h, males from 23.10h until 02.50h.

Univoltine in northern Europe, from the end of May to early July; in southern regions bivoltine, in May and August. In Bulgaria, May/June and July/August (Ganev, 1984). In Navarra Province, Spain, from late April until mid September (Cifuentes, 1997). In the southern Urals, from late May until early July, with a partial second generation in late July\early August (Nupponen & Fibiger, 2002). Late July in northwestern China (Huang & Wang, 2019).
OVUM: Oval (1.75 x 1.40mm) and distinctly dorso-ventrally flattened, glossy, pale olive green (a tint imparted by its gum). Up to 130 may be deposited by each female, usually in pairs, on the underside of the leaves of its hostplant. If a tree is selected, the eggs are laid high in the crown.

LARVA: Full-fed 55--65mm. Dimorphic: green or bluish grey.
On hatching, pale green, about 6mm long and very slender, with a dark tail-horn about one-third of its body-length. Ignoring the eggshell, it wanders about for some time before settling on the underside of a leaf in a characteristic, erect, sphinx-like stance. Feeding normally takes place at night, with growth being confined to an increase in length over the first week; by the first moult most larvae measure c. 11mm.




With further growth, lateral stripes become more and more prominent, changing from light to dark yellow. In the fourth instar small red streaks may appear on the anterior side of these, contrasting vividly with a yellowish or blue-green skin, which is covered in yellow tubercles. The fully-grown larva is noticeably more slender than those of related species, tapering anteriorly to a rather narrow, triangular head. The horn is blue or purple dorsally, red and yellow ventrally and situated above a reddish yellow, tuberculate anal flap.



Prior to pupation, the bright green coloration gradually changes to dull greenish pink on the underside; the red marks fade and the upper surface assumes a greyish brown hue in which the now cream tubercles stand out. The body also shrinks considerably. If touched during this stage, the larva will twitch violently from side to side.
Found throughout July, August and September, although the second brood may feed until October. At times, larvae can be so abundant as to constitute a pest (Albin, 1720; Gninenko et al., 1983; Gninenko, 1998).
Major Hostplants. Many species of Tilia, Ulmus, Alnus and Prunus (cherry, ornamental or wild).
Minor Hostplants. In northern and central Europe, Betula pendula, Quercus, Corylus avellana (Wilson, 1880), Acer, Sorbus (all of which may, in some places, become major hostplants, e.g. Acer pseudoplatanus in Berlin and Sorbus aucuparia in the Alps), Malus, Pyrus and Fraxinus (Caradja, 1893). In southern areas of Europe also on Juglans regia, Castanea sativa and Aesculus hippocastanum (Roueast, 1883). It has also been recorded from mulberries (Morus spp.) (Israel, 1921).
PUPA: 30--35mm. Very dark brown with a reddish tinge, rough and not glossy, unlike most sphingids. Pupation is normally at the base of the host-tree, under a loose mat of grass or, sometimes, damp, dead leaves and flat stones. Bare ground prompts most to wander off elsewhere, although light gritty soil will induce some to burrow 1--2 cm deep. Occasionally, pupae can be found high up in elm trees, wedged into cracks in the bark, or even under loose bark. The overwintering stage.


Ichneumonidae: Callajoppa cirrogaster (Schrank, 1781), Cratichneumon luteiventris (Gravenhorst, 1820), Lymantrichneumon disparis (Poda, 1761), Pimpla illecebrator (Villers, 1789), Pimpla rufipes (Miller, 1759) [syn. Pimpla hypochondriaca (Retzius, 1783)], Protichneumon pisorius (Linnaeus, 1758); Braconidae: Microplitis ocellatae Bouché, 1834, Aleiodes praetor (Reinhard, 1863) [syn. Rogas praetor Reinhard, 1863]; Tachinidae: Blepharipa pratensis (Meigen, 1824), Compsilura concinnata (Meigen, 1824), Drino (Zygobothria) atropivora (Robineau-Desvoidy, 1830), Masicera sphingivora (Robineau-Desvoidy, 1830), Pales pavida (Meigen, 1824), Phryxe vulgaris (Fallén, 1810), Winthemia cruentata (Rondani, 1859), Winthemia rufiventris (Macquart, 1849).
Across central and southern Europe (including Sicily (Mina-Palumbo & Failla-Tedaldi, 1889; Mariani, 1939; Parenzan & Porcelli, 2006; Elisa, iNaturalist 2023)), Kosovo (Bytyçi et al., 2023), the Republic of North Macedonia (Krpač et al., 2019), northern and central Greece (Makrinitsa), western, northern and northeastern Turkey (de Freina, 1979; Ayberk & Akkuzu, 2005; Okyar, Yurtsever, Aktaç & Çakan, 2009; Okyar, 2012; Koçak & Kemal, 2018), Bulgaria (Karisch, 1990), Moldova (Tugulea & Tugulea, 2020) and Ukraine and the Crimea (Savchuk & Kajgorodova, 2015; Khalaim, 2022) to Armenia (Mirzoyan, 1977; Wąsala & Zamorski, 2015) and the Republic of Georgia (Didmanidze, Petrov & Zolotuhin, 2013; Streltzov et al., 2022; Andrey Baznikin, iNaturalist 2023), Daghestan, Russia (Abdurahmanov, 1999; Yakovlev et al., 2022), northern Azerbaijan (Dubatolov, [1999]; Didmanidze, Petrov & Zolotuhin, 2013; Snegovaya & Petrov, 2021) and western Siberia (Eversmann, 1844; Zolotarenko, Petrova & Shiryaev, 1978; Derzhavets, 1984; Izerskiy, 1999; Knyazev, 2020), where it has been found as far east as Tomsk, Novosibirsk, Barnaul, Achinsk, Kulunda, Krasnoyarsk (Stanislav, iNaturalist 2024) and Bijsk (Zolotarenko, Petrova & Shiryaev, 1978; Izerskiy, 1999). There are also several records from the Altai of Russia (Knyazev, 2023) and Kazakhstan (Victor Kolesnikov, iNaturalist 2015), as well as northwestern Xinjiang, China (Huang & Wang, 2019; 'Simbason', iNaturalist 2025). Originally absent from Ireland (Lavery, 1991), but it now appears to be established in Dublin (MothsIreland; Nash & Hardiman, 2018; Connor Helman, iNaturalist 2025). Absent from northern Scandinavia and Arctic Russia, being only recorded as far north as Ob'yacheva (Tatarinov, Sedykh & Dolgin, 2003) and Karelia (Kutenkova, Gorbach, Polevoi & Humala, 2015) in european Russia.
This species has spread farther north in the UK in recent years, with records of it now breeding as far north as Glasgow, Scotland (Payne, 2020; Tracy Murphy, iNaturalist 2025; McGhee, 2025); in 1979 it was deemed as being absent from Scotland (Gilchrist, 1979).
On the Iberian Peninsula this species is recorded from northern Spain (Galicia (Pino Pérez et al., 2009; Fernández Vidal, 2016)) and northern Portugal, with a small population in the mountains of central Spain (Pérez De-Gregorio et al., 2001; Garre, Guerrero, Rubio & Ortiz, 2015). However, Rambur (1942) records a single individual from Malaga, Spain, but it's status in this area requires confirmation.
Wiltshire (1980b) recorded a specimen from Lebanon (collected by A. M. Talhouk), but doubted the authenticity of the locality; however, Talhouk (1997) has confirmed that the record is genuine. As the collection locality was 'Beirut', where the climate and vegetation is most unsuitable for this species, this record is probably the result of an 'import' of a pupa or adult from Europe.
A greater mystery was a 2009 record of a male from northern Pakistan (Great Karakorum Mountains, west of Chikar, 4000–4500m, viii.2009 (leg. V. Gurko, coll. Sphingidae museum of Czech Rep.)). This could have been the result of an 'import' of a pupa or adult from Europe, or a representative of an isolated, relict population. However, as Humairah Hanif et al. (2016) records a further 16 individuals from Chakwal Punjab, this species must be resident; however, these 2016 records require verification. DNA barcode sequence analysis has since confirmed that this population is allied to Mimas tiliae kitchingi Melichar & Řezáč, 2015 from northern Iran.
Extra-limital range. None. Contrary to what is stated in Melichar & Řezáč (2015), this taxon is not present in eastern Russia, where it is replaced by Mimas christophi (Staudinger, 1887).
The Alborz mountains of northern Iran (Sutton, 1963; Lehmann & Zahiri, 2011) and southeastern Azerbaijan (Effendi, 1967; Snegovaya & Petrov, 2021) as the poorly differentiated subspecies Mimas tiliae kitchingi Melichar & Řezáč, 2015. Mimas christophi (Staudinger, 1887) from eastern Russia (Derzhavets, 1984), Japan, northern China (Chu & Wang, 1980b) and Korea (Kim, Nam & Lee, 1982), is a distinct species.
 Return to species list
Return to species list