
Type species: Saturnia pyri ([Denis & Schiffermüller], 1775).
An Holarctic genus comprising about 19 species (including Calosaturnia Smith, 1886), of which seven occur in the region; the others are confined to eastern Asia and California in North America. Closely related to Rinaca Walker, 1855 from the eastern palaearctic, as well as Neoris Moore, 1862 and Perisomena Walker, 1855 [See Rubinoff & Doorenweerd, 2020 for details]. Species of the latter three genera are often included within Saturnia by some taxonomists. In light of recent research, Perisomena may need to be transferred to Rinaca Walker, 1855. Many can be cross-paired in captivity to form hybrids (Nardelli, 2021).
HOSTPLANT FAMILIES: Many trees and shrubs, but with a preference for the Rosaceae, Ericaceae and Oleaceae.
UK: Great Peacock Moth; Giant Emperor Moth; Giant Peacock Moth; Viennese Emperor, F: Grand Paon de Nuit, D: Wiener Nachtpfauenauge; Gro▀es Nachtpfauenauge; Birnspinner, RUS: Pavlynohlazka hrushevaya, S: Större påfågelsspinnare; Stor påfågelspinnare, NL: Grote Nachtpauwoog, CZ: Martináč hruÜňový, H: Nagy pávaszem, E: Gran Pavón, PL: Pawica gruszówka, FIN: Isoriikinkukkokehrääjä, I: Pavonia maggiore, DK: Stor Natpåfugleøje, N: Stor påfuglspinner.
Bombyx pyri [Denis & Schiffermüller], 1775, Ankündung syst. Werkes Schmett. Wienergegend: 49.Type locality: Vienna district, Austria.
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]

Holarctic; western Palaearctic region. Pleistocene refuge: Polycentric -- Holomediterranean refugia (de Lattin, 1967).
Wingspan 87--166mm. The identically marked and coloured sexes are similar to Saturnia atlantica but differ in being larger and in having less concave outer margins to the forewings and areas of chocolate-brown on both wings and body. In Saturnia atlantica these areas are grey and/or black.




This nocturnal species inhabits open landscapes with scattered trees and shrubs, occurring from sea-level in western Europe to over 2000m altitude in the Levant and Iran. Parkland, orchards and vineyards with shade-trees are particularly favoured.
Most adults emerge in the late morning, with females calling that same night, often from the base of trees up which they have climbed. Pairing takes place just before midnight and lasts for about 22 hours. After separation, the male flies off in search of another mate. If possible, the female climbs to the highest vantage point possible before launching herself clumsily towards the nearest shadow on the horizon which, often as not, is a tree. The reason for this strange behaviour is that most females carry too many eggs at first and are 'bottom-heavy'. This stop-start process continues until about 30 eggs have been deposited, usually in chains of five to eight on the trees' branches or trunk. The rest of the eggs are laid on the leaves and twigs of suitable hosts.
Depending on latitude and altitude, late March to the beginning of June as a single generation; however, odd individuals are sometimes found in late autumn (October). In central Europe most are on the wing in mid to late May.
OVUM: Oblong, 2.5 x 2mm, greyish-white with brown gum. Laid in batches of up to ten on the twigs and underside of leaves, usually hatching 14 to 30 days later. Up to 99% of those laid on the trunks and branches of trees can be parasitized.

LARVA: Full-fed 90--100mm. Monomorphic.
The newly-hatched, 5-6mm long larvae consume part of their eggshells and then wander off some distance to find suitable resting sites. At this stage they are mainly black, (sometimes dark brown dorsally) with four longitudinal rows of light-brown tubercles. In the second instar these tubercles become orange. By the third instar the body has become pale greenish-blue, the tubercles bright yellow and the head and anal segments brown. Each tubercle bears a long, black hair, those on the dorsal tubercles being clubbed. Fourth instar and fully-grown larvae are yellowish-green with raised, sky-blue tubercles, with the latter bearing crowns of sharp, liquid-filled spines in addition to the long hairs. Laterally, there is a broad yellow sub-spiracular band on the abdominal segments. Prior to pupation, the entire body changes to golden-brown, against which the blue tubercles virtually light up.
Newly emerged larvae take some time to settle down under a leaf in their characteristic 'U'-shaped positions. Feeding usually consists of eating channels into a leaf. From the third instar onwards many larvae exhibit a kind of 'wanderlust', feeding at one spot for about four days before wandering off along the branches to a new location. This may be a survival strategy, for in it's latter stages great quantities of foliage are consumed, leaving only conspicuous bare twigs.
Larger larvae are capable of 'chirping'. These 'chirps' are broadband, with dominant peaks ranging between the sonic (3.7 kHz) and ultrasonic (55.1 kHz), and are generated by a rapid succession of mandibular 'tooth strikes'. Chirp trains are induced by simulated predator attacks and precede or accompany the secretion of a bitter defensive chemical from integumental spines, supporting the hypothesis that these sounds function in acoustic aposematism. It has been proposed that these caterpillars generate multimodal warning signals (visual, chemical, and acoustic) to target the dominant sensory modalities of different predators, including birds, bats, and invertebrates (Bura, Fleming & Yack, 2009).








Hostplants. Many species of tree and shrubs, with a preference being shown for Persian walnut (Juglans regia). Also pear (Pyrus communis), apples (Malus), sweet cherry (Prunus avium), blackthorn (Prunus spinosa), almond (Prunus dulcis), peach (Prunus persica), olive (Olea europea), ash (Fraxinus excelsior), elms (Ulmus), quinces (Cydonia), limes/lindens (Tilia), birches (Betula), poplars (Populus), willows (Salix), alders (Alnus), horse chestnut (Aesculus hippocastanum), maples (Acer), brambles/raspberries (Rubus), hazels (Corylus), lilacs (Syringa) and planes (Platanus). In Turkey also on the rare Liquidambar orientalis.
PUPA: 35--50mm. Cylindrical, but tapering towards both ends. Blackish-brown with sienna highlights. Formed in a coarse, pear-shaped, double, unsealed greyish-brown cocoon, which is fixed lengthways to the substrate. These are preferentially spun at the base of the hostplant amongst grass growing up to the bark. Bare ground prompts larvae to wander off to find a site on rocks, amongst fallen branches, fence-posts, walls etc. However, a few cocoons can be found high in trees on twigs and branches. Many overwinter two or more times, particularly if the springs are cold.



Ichneumonidae: Metopius dentatus (Fabricius, 1779) [syn. Metopius micratorius (Fabricius, 1804)], Pimpla indra (Cameron, 1899), Pimpla rufipes (Miller, 1759) [syn. Pimpla instigator (Fabricius, 1793)]; Pteromalidae: Stenomalina communis (Nees, 1834) [syn. Pteromalus communis Nees, 1834]; Cynipidae: ?Cynips bombycida Rondani, 1877; Tachinidae: Baumhaueria goniaeformis Meigen, 1824, Carcelia lucorum Meigen, 1824, Chetogena obliquata (Fallén, 1810), Compsilura concinnata (Meigen, 1824), Exorista grandis (Zetterstedt, 1844), Exorista larvarum (Linnaeus, 1758), Exorista rustica (Fallén, 1810), Exorista sorbillans (Wiedemann, 1830), Hubneria affinis (Fallén, 1810), Masicera pavoniae (Robineau-Desvoidy, 1830), Masicera sphingivora (Robineau-Desvoidy, 1830), Senometopia excisa (Fallén, 1820), Winthemia cruentata (Rondani, 1859), Winthemia rufiventris (Macquart, 1849).

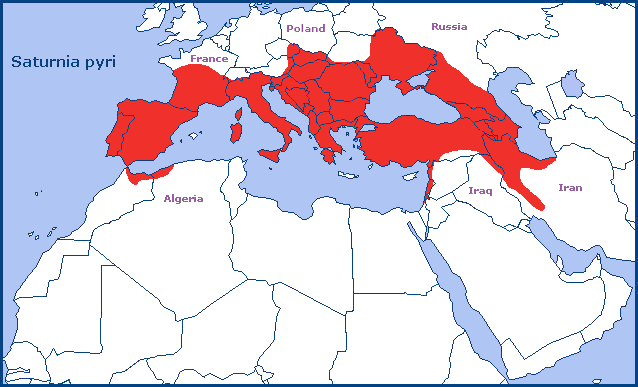
Limited to the warmer areas of Europe and the Near East, from Paris, France (Leraut, 2017), south through western Switzerland, the Iberian Peninsula to costal regions of Morocco and Algeria. Thence eastwards across Italy (Parenzan & Porcelli, 2006), Austria, Czechia, Hungary, the Balkans to the Ukraine (Khalaim, 2022). From here it extends southwards across the Caucasus Mountains (Yakovlev et al., 2022), the Republic of Georgia (Nässig, 1981; Streltzov et al., 2022), Armenia, and Azerbaijan (Zolotuhin, Didmanidze & Petrov, 2011) to Turkey (Nässig, 1981), Syria (Nässig, 1981), Lebanon, Israel, northern Iraq (Kemal & Košak, 2018c) and the Alborz and Zagros Mountains of Iran (Nässig, 1981). There is even a record from central Iran (Moosa, iNaturalist 2020). It is also found on the Mediterranean islands of Corsica, Sardinia and Cyprus, but not Crete.
It now appears to be extinct in northern France (north of Paris), Germany and Luxembourg.
Extra-limital range. None.
None.
 Return to species list
Return to species list