UK: Spanish Moon Moth; Stained-glass moth, F: l'Isabelle; Papillon vitrail; Bombyx Isabelle, D: Isabellaspinner; Spanische Mondmotte; Spanisches Nachtpfauenauge, RUS: Pavlinoglazka Izabella, H: La isabelina spanyol pávaszem; Spanyol pávaszem, E: Mariposa isabelina; Isabela, FIN: Espanjankuukehrääjä; Isabellakehrääjä, I: farfalla luna spagnola, N: Spansk månespinner.
Saturnia isabellae Graëlls, 1849, Revue Mag. Zool. (2)1: 601.Type locality: Spain, Madrid Province, Sierra de Guadarrama, Monte de Pinares Llanos.
(Taxonomic note. A in-depth cladistic analysis of the Actias/Argema/Graellsia species group by Ylla, Peigler & Kawahara (2005) initially demonstrated that Graellsia should be retained as a genus separate from Actias. It appeared to be the most basal of this group, being the first species to split off the Actias/Argema evolutionary line. However, a more recent phylomitogenomic analysis by García-Souto et al. (2025) contradicted this finding. It concluded that the Spanish Moon Moth (Graellsia isabellae (Graëlls, 1849)) clustered monophyletically with the Chinese moon moth (Actias dubernardi (Oberthür, 1897)) and the other Oriental pine-feeding species of this genus, supporting the assertion that Graellsia is a junior synonym of Actias.)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]


Holarctic; western Palaearctic region. Pleistocene refuge: Monocentric -- Atlantomediterranean subsection of the Mediterranean refuge. (Apparently a relict from the Tertiary Period, whose nearest relatives are to be found in eastern Asia.)
Wingspan 65--100mm; bred individuals can be considerably smaller. A pale green species similar in shape and size to the North American Actias luna, but differing in having the wing veins heavily outlined with reddish-brown scales and with yellow replacing the pale green on the hindwing tails and wing submarginal areas. The broad, outwardly curved tails are more than twice as long in males than in the females.








This nocturnal species inhabits mature pine forests at altitudes of 500--1800m, particularly those subject to seasonal low-level cloud cover (i.e. fogs) when the moths are on the wing. Although tolerant of large variations in humidity and temperature, this species cannot tolerate extreme heat or prolonged drought. Both sexes become active at dusk, even at temperatures as low as 5°C. Pairing rarely lasts longer than two hours.
This species prefers to rest near to the forest floor on the stems of saplings and on small branches which, particularly in the male, it 'clasps' with its wings. In this position it is remarkably well camouflaged.
In Canton Valais, Switzerland, it occurs in light pine forest on steep, south-facing slopes at between 800 and 1600m altitude (Heinz Rothacher, pers. comm. 2005).

Depending on latitude and altitude, March to the beginning of July as a single generation.
OVUM: Oval, 2 x 1.8mm, but tapering dorsally. Off-white with dark olive-green speckling. A prominent micropyle is evident. Laid singly or in small groups at the base of young pine needles, usually hatching 10 to 15 days later.

LARVA: Full-fed 70--80mm. Monomorphic.


The newly-hatched, 5mm long larva consumes part of its eggshell. For the first three instars it is mainly greyish-brown with irregular markings and humps, resembling the twigs on which it rests. In most larvae the fourth instar resembles in shape that of Actias selene, but the basic body colour is greyish-brown with some pine-green, brown and yellow irregular blotches: the cervical shield bears four spines. The fifth instar larva is basically apple-green with a broad brown dorsal band edged with white. Each segment has an encircling band of dull red broken by 2-3 white dashes. The thoracic segments are also ringed with yellow, and the ventral surface is reddish-brown, as is the head. Fine white dots cover the entire body, from which a few long brown hairs arise.
Under optimal conditions many larvae will complete their development in four instars, with the final colour pattern appearing in the fourth.


Initially, small larvae rest on pine needles. From the second instar onwards, however, most tend to rest head down at the base of a needle or on an exposed older twig, where they are remarkably well camouflaged. Up until the fourth instar nearly all prefer to eat older needles from the previous year.
The full-grown larva is solitary and often rests with the anterior segments puffed up so as to resemble a pine-cone. In the cool conditions of its habitat it can take up to two months to mature, eating copious quantities of needles in the process. At this stage newer needles may be consumed, as long as they have matured a little. When a pine needle is eaten down to its sheath, older larvae (L4/L5) will seal the resin canals of the stub with silk. This remarkable behaviour is probably due to the danger of becoming entangled in the very sticky resin at the feeding site (Nässig, 1991).
Hostplants. In the wild confined to certain species of Pinus, such as Scots pine (Pinus sylvestris) and the various subspecies of black pine (Pinus nigra) (Marí-Mena et al., 2016); however, in captivity most larvae will also accept Pinus mugo, Picea and Abies. Some will even take Liquidambar as an alternative host, but most will not (Nässig, 1991). In fact, only hybrid larvae of Actias isabellae and species of Actias are known to readily accept Liquidambar.
PUPA: 32--35mm. Mid mahogany brown and similar in shape to that of Saturnia pyri. Formed in a thin-walled, 55 x 30mm, irregular to oblong, single, unsealed, golden-brown, tapered cocoon, which incorporates dead pine needles. These are preferentially spun at the base of the hostplant, either within the needle mat or at the soil/mat interface. Most are orientated horizontally, with the trapdoor through which the moth will emerge situated off-centre on the dorsal surface of the thicker end. Very cold-tolerant, and may overwinter more than one year if the winters are not cold enough.


Ichneumonidae: Coelichneumon orbitator (Thunberg , 1822), ?Coelichneumon microstictus (Gravenhorst, 1829), Exeristes roborator (Fabricius , 1793), ?Ichneumon sulfuripes Rondoni; Tachinidae: Compsilura concinnata (Meigen, 1824), Drino inconspicua (Meigen, 1830), Exorista nova (Rondani, 1859), Exorista segregata (Rondani, 1859), Masicera silvatica (Fallén, 1810), Phaonia tuguriorum (Scopoli, 1763).
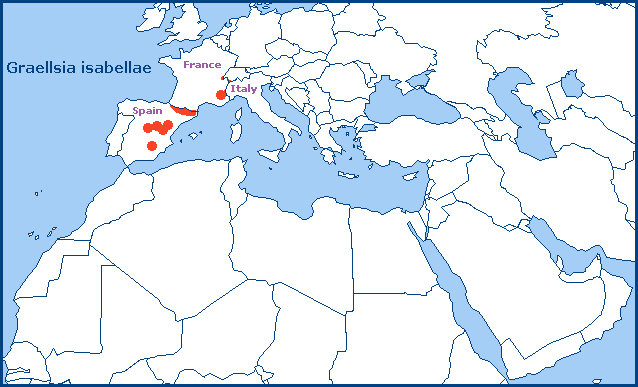
The mountains of central Spain (Romo et al., 2012; Romo et al., 2014; Marí-Mena et al., 2016; Monasterio León, 2017), such as the Sierra de Guadarrama near Madrid, the Montes Universales near Teruel, as well as the Sierra de Javalambre and Sierra Gudar. Also southern Spain in the Segura de la Sierra (Province Jaén (Lara Ruiz, 2011)), parts of Murcia, and the Sierra María-Los Vélez of northern Almeria (Ibáñez, Nevado & Ylla, 2008). It is common in the central and eastern Pyrenees of northern Spain (Vallhonrat et al., 2004; Romo et al., 2012; Romo et al., 2014; Marí-Mena et al., 2016; Monasterio León, 2017), in the Valle del Roncal (Province Navarra) and Sierra de Montgrony (Province Gerona). From here it extends north across Andorra into southern France, where it is restricted to the Pyrénées-Orientales, Ardèche, Drôme, Hautes-Alpes, Alpes-de-Haute-Provence and Alpes-Maritimes, and as a small population near Génissiat, Ain (French Jura) (Marí-Mena et al., 2016).
It has become well established in Canton Valais, Switzerland, since it was first observed/introduced there in 1987/1988 (Heinz Rothacher, pers. comm. 2005; Heiner Ziegler, iNaturalist 2007). It has also been reported from the Aosta Valley of northern Italy (Leraut, 2006), but this remains unconfirmed. There are also recent reports of this species from the Rif Mountains of northern Morocco. The status of this population is unknown.
[This cold-adapted species may have once had a much greater range during the last inter-glacial period. At the end of the last ice-age, when Europe warmed rapidly, Actias isabellae probably got 'stuck' in cool, southern, montane refugia and was thus unable to re-colonize its former more northern range.]
Extra-limital range. None.
None.
 Return to species list
Return to species list