UK: Southern Pine Hawkmoth; Spanish Pine Hawkmoth, F: Sphinx Mauresque; Sphinx des Maures
Hyloicus pinastri maurorum Jordan, 1931, Novit. zool. 36: 243.Type locality: Hammam R'Irha, Algeria.
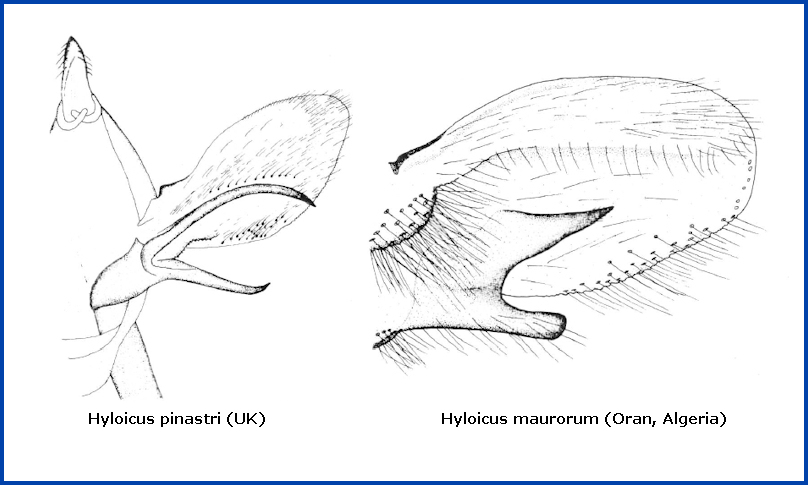
(Taxonomic notes. (i) Eitschberger et al. (1989) raised Hyloicus pinastri maurorum to specific rank but gave no reasons for doing so. However, there are pronounced and consistent differences between the male genitalia of both species, as well as between the eggs, larvae and pupae. Although Hyloicus maurorum and Hyloicus pinastri (Linnaeus, 1758) interbreed in central and eastern France, and have formed natural intermediate populations, this hybrid zone is narrow and stable. It is probable that Hyloicus maurorum evolved for some time in isolation in North Africa and the Iberian Peninsula and that only since the end of the last ice age has it come into contact again with Hyloicus pinastri, which appears to have retreated to south-east Europe during that ice age. Kernbach (1958) reached similar conclusions. These post-glacial zones of secondary contact and hybridization between once isolated sister species are known as suture zones, and have been reported for a variety of taxa in many biomes (Barrowclough et al., 2019; Ipekdal et al., 2020; Moritz et al., 2009; Portnoy & Gold, 2012).
(ii) 'Subsp.' massiliensis is synonymized with Hyloicus maurorum for the same reasons as given for the subspecies of Hyloicus pinastri described by Jordan, namely, that it is a hybrid race between Hyloicus maurorum and Hyloicus pinastri, but one which is closer to the former.)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]
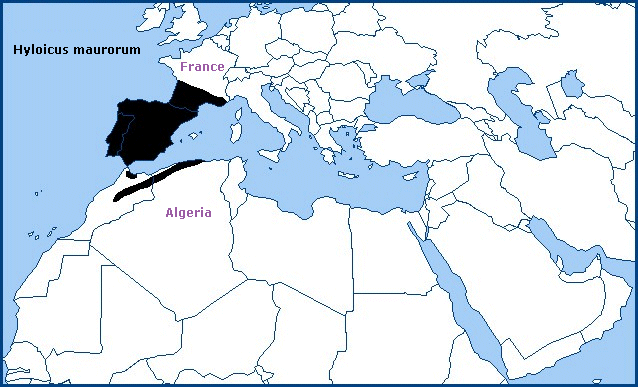
Holarctic; western Palaearctic region. Pleistocene refuge: Monocentric -- Atlantomediterranean/Mauritanian subsections of the Mediterranean refuge.

Wingspan: first generation 70--80mm; second generation smaller. First generation generally indistinguishable superficially from central European examples of Hyloicus pinastri. Second generation often very pale.


In the male genitalia, unlike those of Hyloicus pinastri, both branches of the harpe are short, the upper one flat, pronged, triangularly elongated and pointed (see below right). However, as in Hyloicus pinastri, the apical apophysis and phallus are long.
Apart from its preference for dry pine forests, nothing is known of the adult biology of this subspecies.


Univoltine or Bivoltine. In some years May/June and August as two broods; however, in most years there is only a single brood, with adults mainly on the wing during the second half of July and early August (Cifuentes, 1997; Pittaway, pers. obs.). Odd individuals can sometimes be met with as early as early April or as late as mid September, but this is rare. One was even recorded on 21 February 2022 at Sierra Altaona, Murcia, Spain (John Girdley, pers. comm. 2022).
OVUM: Large, oval and slightly dorso-ventrally flattened (2.1 x 1.75mm); shiny pale yellow at first, but developing a reddish-brown blush at one end after a few days.

Each female lays approximately 100 of these huge, thin-walled and delicate eggs, usually singly in close proximity to each other, on the needles or young twigs of isolated or small groups of trees. Development takes about 10 days. Just before hatching the dark head of the larva becomes visible through the now transparent shell.
LARVA: Full-fed 75--80mm.





On hatching, the larva consumes part of its eggshell. At this stage it is approximately 6mm long and dull pale yellow, although the prolegs, true legs and horn are black, with the latter having a forked tip. The disproportionately large head is mahogany-brown with a pair of near-vertical black streaks on the upper part of the face. With feeding, the body colour changes to olive-green and, after the first moult, six longitudinal creamy-yellow lines appear (three per side), with the ventro-lateral being the broadest. During this stage, the larva sits lengthways on a pine needle and nibbles holes in the surface. Young needles on growing shoots are avoided as they ooze too much sticky resin, which may trap the young larva.
Still disproportionately large, the head is now a paler green than the pine-needle green body and bears a black '/\' mark on the face, which may be edged with white on the outside. The horn and true legs are black, and there is a dot of the same colour on each proleg. Apart from the longitudinal lines becoming broader and more yellowish, and the horn and legs acquiring a wine-red tint, little change takes place to its appearance over the next two moults. It now starts to consume whole needles, which are grasped between the true legs and eaten from the tip down.
Towards the end of the fourth instar the green areas of the body may become speckled with white and the black streaks on the face may be replaced with reddish-brown. The dark, transverse divisions of each segment, so characteristic in the final instar, also start to appear.
Immediately after the final moult the larva resembles that of Hyloicus pinastri, in being basically green with a brown dorsal band, dark transverse divisions to each segment and broken longitudinal white lines. However, as the cuticle hardens, all trace of green is lost. Fully grown, the larva is rather slim and of equal thickness throughout its length. The thin, slightly curved and granular horn is black. The large head is ochreous yellow with a brownish blush on the face, particularly in the lower area. The black lines of the face, so evident in the early stages, disappear in most individuals, to be replaced by black cheeks. The intersegmental cuticle behind the head is black. This is prominently exposed if the larva is 'attacked', probably in imitation of the band of poisonous black hairs found in the pine-feeding lasiocampid Dendrolimus pini (Linnaeus, 1758). (This behaviour can also be found in the Californian Sphinx perelegans Henry Edwards, 1874, which has violet intersegmental cuticle and a pair of eye-like black spots on top of the head.) The shield on the first abdominal segment sports longitudinal yellow and black bands, and the spiracles are orange.


So far, this description is similar to that of Hyloicus pinastri; however, in Hyloicus maurorum the basic body colour is medium grey. Dorsally, this is suffused with brown so that the brown dorsal band is no longer distinct. The body segments are distinctly separated one from another, and each segment in turn is subdivided by sunken, dark, transverse lines so that the entire body appears to consist of numerous narrow rings joined together. As in Hyloicus pinastri, the longitudinal white lines are now fragmented into streaks. On the posterior edge of each segment, and between the white 'lines', there appears a rectangular black dot. The body is smooth, but not glossy, unlike the head, underside, legs and shield. However, an oily appearance extends over the entire body as the larva darkens prior to pupation.
Throughout its entire life the larva is very well camouflaged. Although young larvae rest along pine needles, the fully grown larvae prefers to snuggle down at the base of those needles along the twig, with which it blends.
Major Hostplants. Pinus spp., especially Pinus halepensis and Pinus pinaster.
Minor Hostplants. Cedrus.
PUPA: As Hyloicus pinastri, but with a slightly shorter projecting tongue case. May diapause two or three years, especially when the spring is cold or late. The overwintering stage.


Tachinidae: Drino (Zygobothria) atropivora (Robineau-Desvoidy, 1830).

The Iberian Peninsula, including both the north and south slopes of the Pyrenees; southern and central France as far north as Corrèze (Haxaire, 2009), and the Atlas and Rif Mountains of North Africa (Rungs, 1981). Its discontinuous distribution is due to a preference for dry pine forests at higher altitudes (to 2000m).
The situation in France appears to have changed over the last 20 years, with this species displacing Hyloicus pinastri from many southern areas. True Hyloicus pinastri can now only be found north of Chateauroux, in the Alps and Var. Populations around Toulon are clearly intermediate hybrids (J. Haxaire & J.-M. Bompar, pers. comm.).
The population on Corsica (Bretherton & de Worms, 1963) has been classified at various times as Hyloicus pinastri or Hyloicus maurorum (Haxaire, 2009; Haxaire, pers. comm 2018; Haxaire, 2019). However, this population has now been reclassified as Hyloicus corsica Haxaire, Melichar & Rougerie, 2023.
Extra-limital range. None.
None.
 Return to species list
Return to species list