UK: Small Elephant Hawkmoth, F: Petit Sphinx de la Vigne; Petit Pourceau, D: Kleine Weinschwärmer, RUS: Rozovyi malyi Brazhnik, S: Liten Snabelsvärmare; Mindre Snabelsvärmare, NL: Klein Avondrood, CZ: Lišaj kyprejový, H: piros szender, PL: Zmrocznik pazik, FIN: Pikkukiitäjä, I: Sfinge porcellino, HR: mali vinski ljiljak, DK: Lille vinsværmer, N: Liten snabelsvermer, EST: Väike-punasuru.
Sphinx porcellus Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 492.Type locality: Unspecified [Europe].
(Taxonomic note. The status of Deilephila porcellus and all its forms (including 'subsp.' suellus) presents some taxonomic problems. Many regard some, particularly suellus, as valid (sub)species; however, all are almost certainly forms of Deilephila porcellus (see also Kernbach, 1958; Ebert, 1976; de Freina, 1979), which diverged from the parent stock in isolated refugia during the last ice age, and all are treated as such in this work. 'Subsp.' porcellus and suellus have since come together again and are now intergrading in Turkey, northern Iran and the Tian Shan of Central Asia. Further evidence for the non-subspecific status of 'subsp.' suellus is supplied by temperature-breeding experiments with 'subsp.' porcellus: dry heat applied to developing pupae of British 'subsp.' porcellus can result in adults which are externally almost identical to those of 'subsp.' suellus. There are also no differences between the male genitalia and early stages of 'subsp.' porcellus and 'subsp.' suellus (de Freina, 1979). For these reasons, all attempts to describe 'subspecies' of porcellus should be abandoned.)
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]

Holarctic; western Palaearctic region. Pleistocene refuge: Poly/monocentric -- Atlantomediterranean, Adriatomediterranean and Pontomediterranean subsections of the Mediterranean refuge. Also, the Syrian, Iranian and Turkestan refugia, with slightly different forms evolving in each. These have since started to merge with each other to produce additional forms.

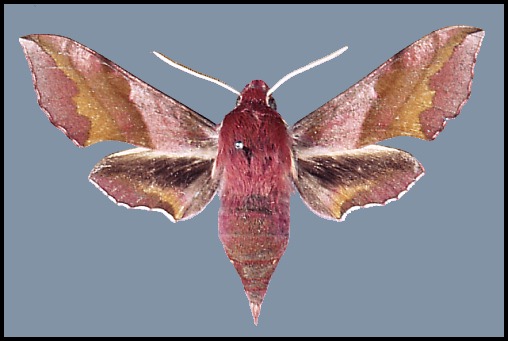
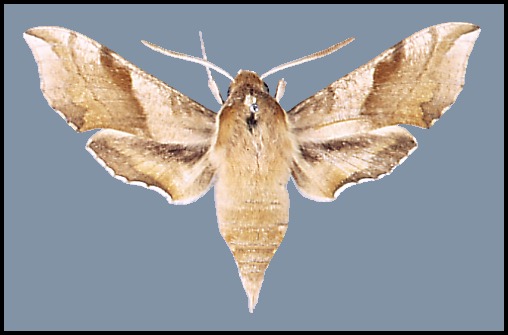
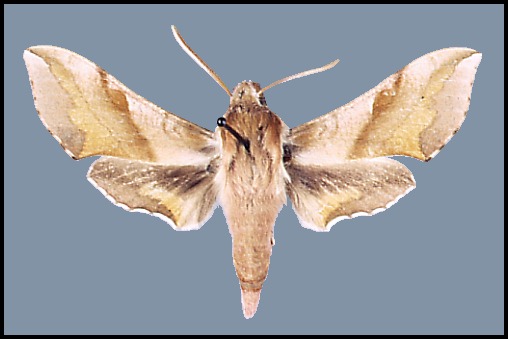
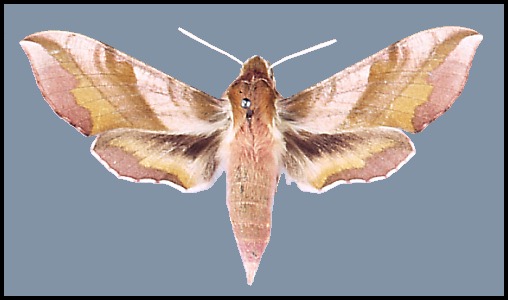
Wingspan: 40--62mm. A distinctive species over most of its range, but highly variable in colouration. In drier and warmer areas of Asia Minor and Central Asia many individuals/populations loose some if not all of their pink colouration: intermediate forms are f. rosea Zerny. In f. indistincta Tutt, the red is replaced by pinkish grey. In f. suellus all pink coloration is replaced by a yellowish sandy buff. Such examples tend to be more frequent in arid areas.






Individuals from northwest Africa, as well as from Lebanon, Israel and the Toros Mountains of southern Turkey, may be larger than average and more brightly coloured. These two populations appear to be related and may represent the western and eastern remnants of a population which once occurred right across North Africa during the last ice age.
Frequents coastal areas, heaths and meadowland, especially uncut field verges and roadsides where Galium is present, usually in local colonies, where it may be abundant. Up to 1600m in the Alps and Spain (Pérez De-Gregorio et al., 2001), but in North Africa, Turkey and northern Iran it is restricted to northward-facing mountain slopes at high altitudes, generally at 1500--2000m (Ebert, 1976).
In central Iran and Central Asia frequents open, dry montane forest, or scrub with a good ground cover of herbs. Usually found at about 2500m, rarely below 2000m.

Although its general coloration is very noticeable against an artificial background, amongst low vegetation, where it rests by day, it breaks up the outline of the moth very well. At dusk, Deilephila porcellus sallies forth in search of flowers such as Valeriana, Iris, Salvia, Echium, Silene and, especially, Rhododendron, ceasing to feed 2--3 hours later. After midnight, the species is once again active, but this time in search of a mate. Pairing takes only a short time, after which females alternate feeding with egg-laying as soon as dusk falls, depositing their small green ova amongst Galium shoots whilst hovering. Readily attracted to light, large numbers often arriving in a short space of time.
In Europe late May to early July, depending on the weather, and, in southern Europe, again in August. In North Africa late April/May/June, depending on the location; the Moroccan populations mainly in mid-June. In the southern Urals, from late May until mid July (Nupponen & Fibiger, 2002).
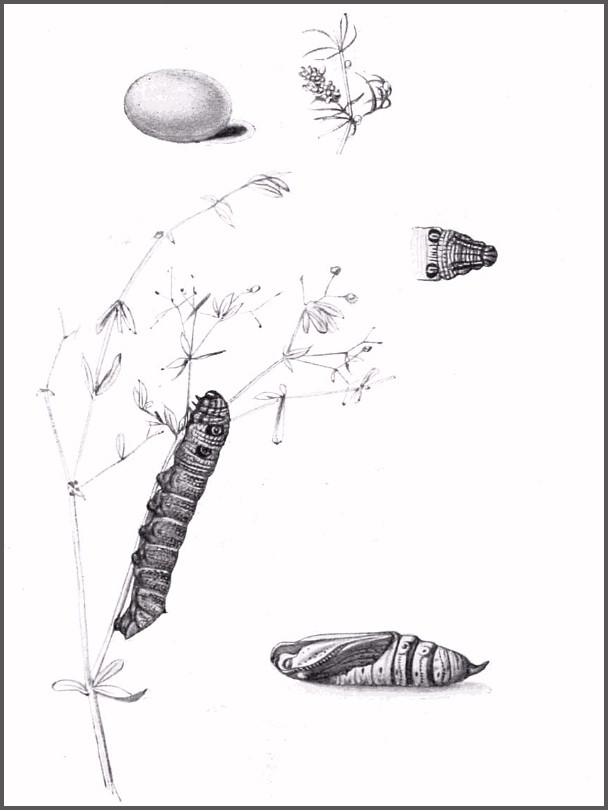
OVUM: Small (1.20 x 1.05mm), oval, with a dorsal depression, clear green. Changes to yellowish green before hatching. Laid singly near the growing tips of the hostplant, often two or three to a clump of shoots.
LARVA: Full-fed, 55--70mm. Dimorphic: brown and green.






On hatching, the larva is approximately 3mm long, pale greyish green, with a tinge of yellow ventrally and two small tubercles in place of a tail horn. In the second and third instars the primary green colour darkens, a pale dorso-lateral line appears and the caudal tubercles are pink. In the next moult the eye-spots and a dark bloom develop on the body, the bloom becoming dominant in the final instar when most assume a grey-brown coloration; only a few remain green. If the larva is alarmed at this stage, it retracts its head and thoracic segments causing the eye-spots on abdominal segment 1 to become prominent. If this fails to deter the 'attacker', Deilephila porcellus then feigns death, becoming completely limp and flaccid. Fully grown, nearly all feed by night, resting during daylight hours low down on the hostplant. Although a mixed diet of flowers and leaves is initially preferred, when feeding on Galium, many make do with leaves only with no ill-effects. Prior to pupation the skin darkens, an event more noticeable in the rare green form; the larva may then travel a considerable distance in search of a suitable pupation site.
A study of the very detailed description given by Degtyareva & Shchetkin (1982) of a larva of Eumorpha suellus gissarodarvasica Shchetkin (= Deilephila porcellus 'suellus') taken in the Darai-Nazarak Gorge, Gissar Mountains, Tajikistan, reveals no discernible difference between the larva of Deilephila porcellus 'suellus' and that of Deilephila porcellus 'porcellus'.
Most common between July and September.
Major Hostplants. Galium spp.
Minor Hostplants. Rarely on Epilobium, Impatiens, Asperula, Lythrum, Vitis and Parthenocissus. It used to be a minor local pest of grapevines in southern Europe (Picard, 1920).

PUPA: 25--31mm. Light brown with streaks and dashes of darker brown; prominent eyes, a cariniform proboscis and a pointed, triangular, downward-curving cremaster. As in Deilephila elpenor (Linnaeus, 1758), there is a semi-circle of small hooks on each movable abdominal segment. Pupation takes place in a loosely spun cocoon amongst litter on the ground or sometimes under a stone. The overwintering stage.


Ichneumonidae: Amblyjoppa fuscipennis (Wesmael, 1845), Amblyjoppa proteus (Christ, 1791), Banchus falcatorius (Fabricius, 1775), Banchus palpalis Ruthe, 1859, Barylypa insidiator (Förster, 1878), Callajoppa exaltatoria (Panzer, 1804), Callajoppa cirrogaster (Schrank, 1781), Goedartia alboguttata (Gravenhorst, 1829), Ophion luteus (Linnaeus, 1758), Pimpla rufipes (Miller, 1759), Protichneumon pisorius (Linnaeus, 1758); Tachinidae: Drino (Zygobothria) atropivora (Robineau-Desvoidy, 1830), Drino lota (Meigen, 1824), Drino vicina (Zetterstedt, 1849), Masicera sphingivora (Robineau-Desvoidy, 1830), Oswaldia spectabilis (Meigen, 1824), Phryxe vulgaris (Fallén, 1810), Thelaira nigripes (Fabricius, 1794), Winthemia quadripustulata (Fabricius, 1794).


With the exception of the southern half of the Iberian Peninsula (Pérez De-Gregorio et al., 2001) and northern Scotland and northern Scandinavia, widespread throughout the region, from Ireland (Lavery, 1991) and Galicia (Pino Pérez et al., 2009; Fernández Vidal, 2016) and the rest of western Europe (Newman, 1965), including Sardinia (Parenzan, 1995; Parenzan & Porcelli, 2006), Sicily (Parenzan, 1995; Parenzan & Porcelli, 2006), Greece (Weidlich, 2016), Albania (Rebel & Zerny, 1932), the Republic of North Macedonia (Krpač et al., 2019) and Bulgaria (Karisch, 1990), to Novosibirsk, Barnaul, Tomsk and Krasnoyarsk in western Siberia (Eversmann, 1844; Zolotarenko, Petrova & Shiryaev, 1978; Izerskiy, 1999), eastern Kazakhstan (Zolotarenko, Petrova & Shiryaev, 1978; Toropov, Milko, Zhdanko & Evdoshenko, 2023) and Kyrgyzstan (Toropov, Milko, Zhdanko & Evdoshenko, 2023). Recorded as far north as Karelia (Kutenkova, Gorbach, Polevoi & Humala, 2015), Arkhangelsk Oblast (Kozlov, Kullberg & Zverev, 2014), and the Biostantsiya Syktgu (Tatarinov, Sedykh & Dolgin, 2003) in european Russia.
Then south from the Chinese Tian Shan and other parts of Xinjiang Province, China (Chu & Wang, 1980a; 1980b; Pittaway & Kitching, 2000), across southern and eastern Kazakhstan (Staudinger & Rebel, 1901; Eitschberger & Lukhtanov, 1996; Shovkoon, 2015), Kyrgyzstan (Bang-Haas, 1927; Dubatolov, [1999]; Korb, 2018), the Zeravshan Mountains of Tajikistan (Alpheraky, 1882; Bang-Haas, 1927; Degtyareva & Shchetkin, 1982; Shchetkin, 1975), southern Uzbekistan (Danner, Eitschberger & Surholt, 1998; Omonov et al., 2025), southern Turkmenistan (Danov & Pereladov, 1985; Danner, Eitschberger & Surholt, 1998), the Crimea (Efetov & Budashkin, 1990) to northern and eastern Iran (Ebert, 1976; Lehmann & Zahiri, 2011; Didmanidze, Petrov & Zolotuhin, 2013), north-eastern Iraq (Wiltshire, 1957), 2005b), the Caucasus (Bang-Haas, 1938; Derzhavets, 1984), the Republic of Georgia (Didmanidze, Petrov & Zolotuhin, 2013; Streltzov et al., 2022), Armenia (Ebert, 1976; Didmanidze, Petrov & Zolotuhin, 2013; Wąsala & Zamorski, 2015) and Azerbaijan (Didmanidze, Petrov & Zolotuhin, 2013; Snegovaya & Petrov, 2021), and northern Turkey (Ebert, 1976; de Freina, 1979; de Freina, 2012; Didmanidze, Petrov & Zolotuhin, 2013; Kemal & Koçak, 2014; Koçak & Kemal, 2018).
The isolated population in North Africa is restricted to the Middle Atlas and Rif Mountains of Morocco (Ifrane, Jaba Forest, Mischliffen, Dayet-Aaoua, Ketama, etc. (Rungs, 1981)), and the Atlas Mountains of Algeria and western Tunisia (Oberthür, 1916).
There is also another similar-looking, isolated population in the Lebanese mountains (Zerny, 1933; Ellison & Wiltshire, 1939), northern Israel (Bytinski-Salz, 1966; Müller et al., 2005b) and Toros Mountains of southern Turkey. This may be the eastern remnant of a population which once occurred right across a cooler, greener and wetter North Africa during the last ice age. The Atlas Mountains population is the western remnant.
Extra-limital range. Central Siberia, the Altai Mountains of Russia and Mongolia (Gus’kova & Yakovlev, 2011; Yakovlev & Doroshkin, 2017; Makhov, Matov & Lukhanov, 2024), Yakutia/Sakha (Kaimuk et al., 2005), Lake Baikal (Derzhavets, 1984), Transbaikalia (Oleg Korsun, pers. comm. 2004), Amurland (V. Dubatolov, pers. comm. 2012) and neighbouring Inner Mongolia, China (Ma, Li & Kang, 1991).
 Return to species list
Return to species list