Type species: Smerinthus davidi Oberthür, 1884.
A monotypic genus confined to the central-southern Palaearctic.
IMAGO: Apex of forewing pointed; outer margin sinuate, convex. Frenulum and retinaculum present. Proboscis extending to beyond middle of abdomen. Labial palpus short, not porrect. Pendant scales (lashes) present at upper edge of eye. Male antenna with long seriate setae; female antenna simply cylindrical without lateral grooves or prolonged setae; terminal segment in both sexes short. Transverse head crest between antennae. Tibiae without spines, but foretibia with long apical thorn; hindtibia with two pairs of spurs. Pulvillus small. Paronychium present, but ventral lobe vestigial. Veins Rs and M1 of hindwing stalked; M2 from middle of cell.
Genitalia. In male, valva broadly sole-shaped without friction scales on outer side; apex rounded. Sacculus represented by a small sub-basal, broadly triangular ridge, which bears an obliquely vertical carina. Phallus without any special armature, but with apical edge produced on one side. In female, lamellae weakly chitinized with no special armature.
OVUM: Large, oval and yellowish-white.
LARVA: Head tiangular; body cylindrical, green, granulose, with seven yellow oblique lateral stripes. Horn erect and the same colour as the body stripes.
PUPA: Dark mahogany brown, glossy, with a very sharp, pointed cremaster. Very like that of Proserpinus proserpina (Pallas, 1772) in shape and colour.
HOSTPLANT FAMILIES: Anacardiaceae.
UK: Pistacia Hawkmoth
Smerinthus davidi Oberthür, 1884, Bulletin Bimensuel of the Bull. Soc. ent. Fr. 1884(2): 14.Type locality: Asia Minor.
(Taxonomic notes. (i) The volume and part number, as well as the pagination, is that of the Bulletin Bimensuel of the Bull. Soc. ent. Fr. published on 23 January 1884, rather than that of the quarterly version published on 31 July 1884 with different (Roman numeric) pagination (see Evenhuis, 2002; STI 22552). This species was proposed again by Oberthür, 1884, Études ent. 9: 29.
*(ii) Based on phenotypic and genitalial differences, specimens of the nominate subspecies (Akbesia davidi davidi) from southeast Republic of Georgia, southern Armenia, Azerbaijan and the western Alborz Mountain range (Iran) were separated out as a new subspecies, namely Akbesia davidi hofmanni De Freina & Geck, 2018. These exhibited some characters that indicated a closer relationship to Akbesia davidi gandhara. However, although some individuals from northern Iran and Azerbaijan resembled this latter subspecies (De Freina & Geck, 2003; 2018), others appeared to be intermediate between Akbesia davidi gandhara and Akbesia davidi davidi. This region may thus represent a suture zone between what were formally separated populations.)
Holarctic; western Palaearctic region. Pleistocene refuge: Monocentric -- Iranian refuge. Possibly also Syrian refuge.

Wingspan: 60--70mm. A very striking species. Difficult to confuse with any other in the western Palaearctic. Shows little variation in coloration; however, the beautiful green colour of the forewings starts to fade rapidly after a few days, and can almost vanish in older individuals. Also, in some individuals the grey areas on wing and body can be flushed with bright pink.


Although a local species, it often occurs in large numbers at certain sites in rocky, hilly areas supporting scattered trees and shrubs of Quercus, Olea, Ceratonia and Pistacia. Attracted to light, often in large numbers. Males are generally on the wing before females, with both mainly active after midnight, often just before dawn. Although the adults do not normally feed, they will drink water if offered (Danner, Eitschberger & Surholt, 1998), but in captivity they imbib very little (De Freina & Geck, 2003). The only known nectar source recorded in the wild is a blue-flowered species of Linum (Naumann & Zolotuhin, 2000).


Bivoltine; in Iran, April/May and late July/August, but across northern Iraq during May/June and August (Wiltshire, 1957). Two specimens were taken at Feke (Adana Province, 37°50'N, 35°53'E, 625m), Turkey, on 21 June 2001 (Feza Doganlar, pers. comm.). Early to late June in Jordan, depending on altitude (Müller et al., 2005a). Early June has also been recorded for Armenia (Wąsala & Zamorski, 2015). From Iranian Beluchistan there are records for April (Barou, 1967) and early June (Daniel, 1961).
OVUM: Large for the size of moth (1.55 x 1.90mm), oval, pale lime-green to start with, changing to yellowish-green after a few days, then whitish-yellow just before hatching (De Freina & Geck, 2003). Three females kept in captivity laid between 88 and 128 eggs each, mostly over five nights (De Freina & Geck, 2003). This stage lasts about 7 days under natural conditions.
Shrubs less than 3 meters high and growing in sunny locations are generally selected for oviposition, with a preference being shown for more exposed branches growing up against hot rock faces or over bolders (De Freina & Geck, 2003). Eggs are laid singly on the underside of the hostplant's leaves (Danner, Eitschberger & Surholt, 1998), with fully-formed new leaves preferred (De Freina & Geck, 2003). Immature, undeveloped and older leaves are avoided (De Freina & Geck, 2003).
LARVA: Full-fed: 43-45mm.
On hatching the 6mm-long, whitish larva, with its long, pale reddish-yellow horn, wanders off to find a leaf under which it spins a silken pad on which to moult; the discarded egg case is ignored (De Freina & Geck, 2003). Not until the second instar does normal feeding commence from beneath that leaf, but only at night. It then becomes greenish-yellow with dark green dorsal stripes. Both body and head are covered with fine tubercles. Over the next two instars the larva becomes bluish-green with yellowish-white oblique lateral stripes. The latter drop down from a broad, pale, dorso-lateral band which runs from the base of the horn up onto the head, terminating at the base of two apical, rose-colored tubercles. Horn rose-colored, anal flap edged yellowish-white. Legs the same basic green colour as the body, but often tinged yellowish-white and/or rosy (Danner, Eitschberger & Surholt, 1998). During these stages it tries to avoid direct sunlight (De Frein & Geck, 2003).
Two main colour forms are evident in the final instar, namely plain bluish-green and bluish-green with salmon-red patches surrounding the spiracles and another at the base of the last pair of true legs. A pale yellow sub-spiracular line runs from the head to this first salmon patch, and there are seven oblique lateral stripes of the same colour. The cheeks of the triangular head and the horn are also of the same shade of yellow. The false abdominal legs are yellow at their base, with salmon-red 'socks'. Fully grown it looks similar to that of Mimas tiliae (Linnaeus, 1758). There are also much rarer pinkish-brown and yellowish-green forms (De Freina & Geck, 2003).
[Considerably more information on the larval stages can be found in De Freina & Geck (2003).]







Hostplants. Larvae have been found on Pistacia atlantica (Danchenko, Zarikian & Sargsyan, 2017), Pistacia palaestina (De Freina & Geck, 2003; Müller et al., 2005a) and Pistacia terebinthus (Vyacheslav Ivonin & Yanina Ivonina, pers. comm. 2022), and will probably be found to also feed on Pistacia lentiscus. In captivity most larvae will eat Cotinus coggygria and Rhus coriaria, indicating that these shrubs may be natural hosts in the wild (De Freina & Geck, 2003). Newly hatched larvae will commence feeding on the North American Rhus typhina, but fail to thrive on this hostplant (Vladimir Kiselev, pers. comm. 2015).
In 2021 numerous larvae were found on Pistacia terebinthus around Silifke and Erdemli, Mersin Province, southern Turkey, on shrubs growing both in open, rocky areas and among open pine forest (Vyacheslav Ivonin & Yanina Ivonina, pers. comm. 2022).


PUPA: 30mm. Dark mahogany brown, glossy, with a darker, very sharp, pointed cremaster. Very like that of Proserpinus proserpina. Formed in a loose cocoon among debris on the ground. The overwintering stage.
About 2% of first generation pupae do not produce a second generation and go straight into diapause (De Freina & Geck, 2003).
Tachinidae: Drino (Zygobothria) atropivora (Robineau-Desvoidy, 1830).

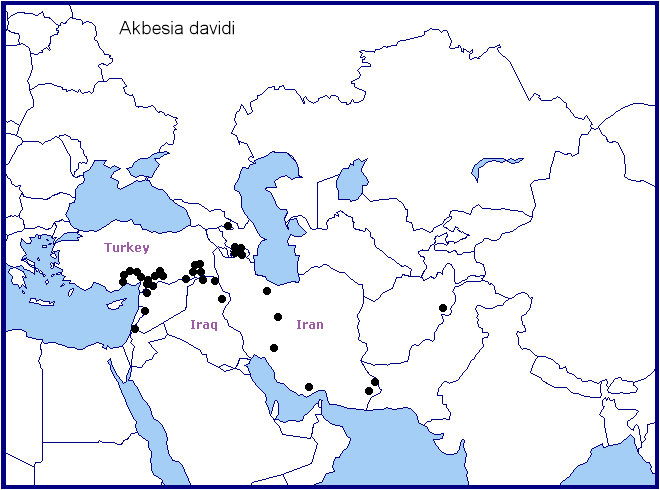
Recorded sporadically from central-southern and south-eastern Turkey (Oberthür, 1884; Daniel, 1939; De Freina & Geck, 2003; Feza Doganlar, pers. comm.; Kemal & Koçak, 2016; Koçak & Kemal, 2018; Kemal, Koçak & Uçak, 2018; Seven, 2020; Vyacheslav Ivonin & Yanina Ivonina, pers. comm. 2022; Seven & Aykal, 2022), northern Syria (e.g. 12km SW Qamishli) (De Freina & Geck, 2003), northern Israel (D. Benyamini, pers. comm.; Müller et al., 2005b), western Jordan (Müller et al., 2005a), north-eastern Iraq (Wiltshire, 1957; Kemal & Koçak, 2018c; SarWar, iNaturalist 2022), southeastern Republic of Georgia (Naumann & Zolotuhin, 2000; Didmanidze, Petrov & Zolotuhin, 2013; Valentinus Tikhonov, iNaturalist 2014), Armenia (Wąsala & Zamorski, 2015; Danchenko, Zarikian & Sargsyan, 2017; Karagyan, Kalashian, Ghrejyan & Danchenko, 2019), Azerbaijan (Snegovaya & Petrov, 2021), northern Iran (Alborz Mountains) (De Freina & Geck, 2003; Lehmann & Zahiri, 2011), the Zagros Mountains of western Iran (Boyer-Ahmad, Kohgiluyeh Province, 20-24.v.2011; Ghassemi, Alemansoor & Alehossein, 2010) and Iranian Beluchistan (Daniel, 1961; Barou, 1967).
Akbesia davidi davidi may also occur (more widely) across Azerbaijan, the Alborz Mountains of northern Iran and southern Iran, as the vegetation zones in these areas are similar to those where it is found farther west.
This uncommen species is named after the town of Akbez (near Hassa) in Turkey (N.E. of Iskenderun). As much of the original vegetation has been destroyed in this and many other regions farther east, it is possible that Akbesia davidi davidi may have become extinct over some of its former Turkish range. Additionally, as the larvae feed on Pistacia, the extensive cultivation of pesticide-treated groves of Pistacia vera may have contributed to this species decline.
Extra-limital range. None.
Eastern Afghanistan (Sarobi) (Ebert, 1969) as Akbesia davidi gandhara De Freina & Geck, 2003, which may also occur in other areas of Afghanistan and into Pakistan.
 Return to species list
Return to species list