Type species: Samia cynthia (Drury, 1773).
A genus of maybe 12 closely related species from the eastern Palaearctic and Oriental regions, with the base species/race being Samia watsoni Oberthür, 1914 (Du, Huang, Naumann, Kitching, Xu, Sun & Wang, 2022). One, Samia cynthia, has been introduced into several other parts of the world as subsp. cynthia. [Note: Many lepidopterists still regard Samia as consisting of one very variable species (or two/three).]
The Himalayan Samia canningi (Hutton, 1859) has been demonstrated to be the wild progenitor of the closely related and domesticated Eri Silkmoth (Samia ricini (Jones, 1791)), which exists primarily in captivity (Du, Huang, Naumann, Kitching, Xu, Sun & Wang, 2022). Therefore, the two names refer to the same biological species, but the name Phalaena ricini Jones, 1791 has precedence over Saturnia canningi Hutton, 1859. However, both names have been used widely and consistently by authors in the entomological and sericultural literature for over 150 years to refer to the domesticated and wild entities, respectively. Peigler & Luikham (2013) proposed that the name Saturnia canningi be conserved and added to the Official List of Specific Names in Zoology, so that it can continue to be used when referring to the wild form (Peigler & Luikham, 2013; ICZN, 2016). However, further DNA analysis has shown that Samia ricini is a polyhybrid that was created in captivity, and that it also contains the genes of several other Samia cynthia races/subspecies.
HOSTPLANT FAMILIES: Mainly trees of the genus Ailanthus (Simaroubaceae), although some species prefer other hosts.
UK: Cynthia Moth; Ailanthus Silkmoth, F: Bombyx de l'Ailanthe, D: Ailanthusspinner; Götterbaumspinner, RUS: Aylantoviy selkopryad, NL: Hemelboomvlinder, H: Bálványfa-selyemlepke, FIN: Ailanthuskehrääjä, I: Bombice dell'ailanto, N: Gudetrepåfuglspinner.
Phalaena (Attacus) cynthia Drury, 1773, Illustr. Exot. Ins. 2: 10.
Type locality: China.
[Further details on this species, as well as photos of all stages, can be found on Lepiforum.]
Holarctic; eastern Palaearctic region. Pleistocene refuge: Monocentric -- Sinopacific refuge.
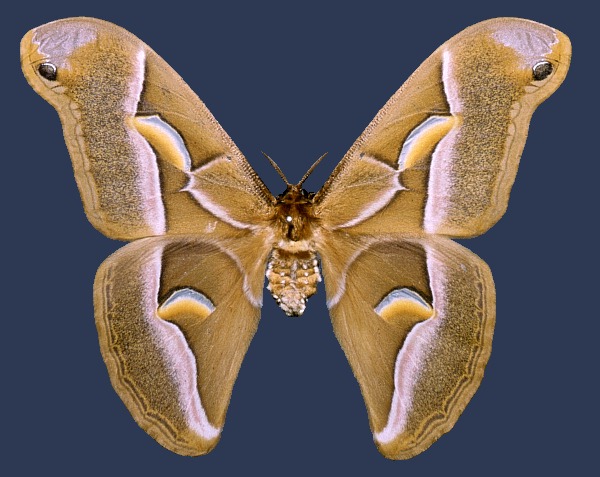
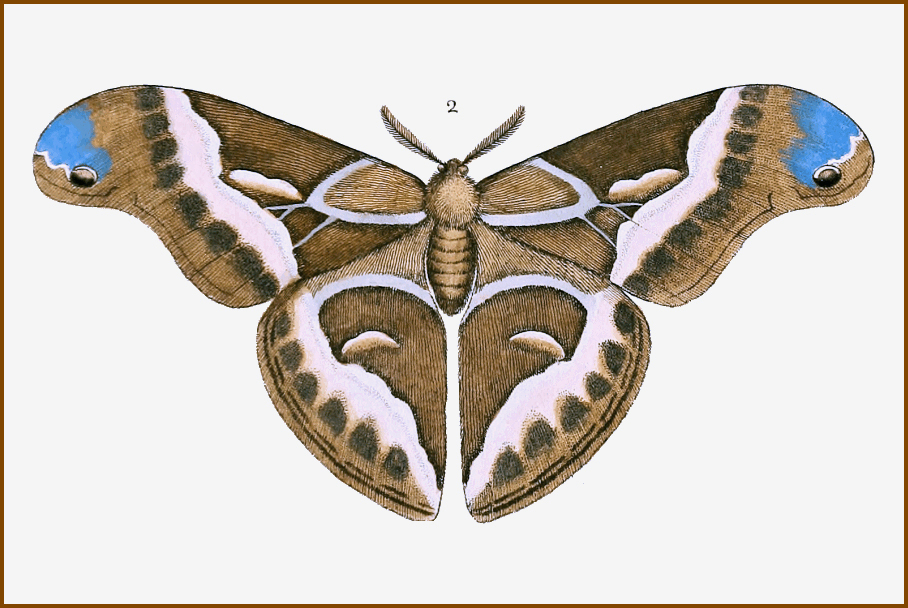
Wingspan 113--125mm. Cannot be confused with any other European saturniid, resembling most closely the genera Callosamia (North America), Epiphora (Africa) and Attacus (South East Asia). Body small (<25mm) in relation to wingspan. Male forewings more falcate than in the plumper female, otherwise the sexes are identical, including the 'snake-head' pattern to the forewing tip. The eyespots on each wing are not round but squashed into large crescents. Somewhat variable, with f. advena Watson, 1912 having a reduced grey post-apical area, and f. parisiensis Clément, 1899 with yellowish-brown ground colour.




Although originally from the open lowland forests of eastern and central China, in Europe this nocturnal, or even crepuscular, species is associated mainly with cities and suburban areas, where its introduced host tree is grown as an ornamental or has become feral. It is rarely found above 400m altitude.
Most adults emerge in the late morning, with females calling that same night, or even during late afternoon. Pairing usually takes place just after sunset and lasts for up to 12 hours. Thereafter the female deposits up to 400 eggs on the underside of the hostplants' leaves. By day the adults rest among foliage.
A sedentary, non-dispersive species, with females very attached to the tree or thicket of saplings where they emerged. Most eggs will be laid within this location. Males are more adventurous and will fly some distance in search of females.
In northern Europe during May and June as one generation. In southern Europe a partial second generation may occur in September.
OVUM: Oblong, 1.5 x 1mm, greyish-white with brown gum. Laid in quite large, crescent-shaped batches on the twigs and underside of leaves, usually hatching 10 to 20 days later.

LARVA: Full-fed 65--80mm. Monomorphic.


The newly-hatched, 4mm long larvae consume part of their eggshells. At this stage they are mainly yellow with black-tipped, conical tubercles, black legs, and a black head which is carried almost horizontal. The body bears longitudinal rows of black spots. In the second instar the body colour becomes paler, with the cervical shield reduced to a pair of black stripes. In the third instar the head becomes retractible, the body pale yellow, the dorsal and dorso-lateral tubercles white. These tubercles loose their black tips but little black areas appear on the anal claspers and flap. By the fourth instar the head has become pale brown, the yellowish-green body becomes covered with a white, powdery bloom, and the legs turn chrome yellow. In the final instar some of the body ridges become bluish and the head greenish. Under the powdery bloom, the body is a pale bluish-green.


Newly emerged larvae quickly settle down under a leaf in groups, where feeding usually consists of eating channels into a leaf. Only in the third or fourth instar do they become solitary.
Hostplants. Almost exclusively on the introduced Chinese Tree of Heaven (Ailanthus altissima (Mill.) Swingle), which is grown as a city ornamental and which has also established itself in many parts of central and southern Europe. Larvae have also been found on Forsythia, ashes (Fraxinus), Persian walnut (Juglans regia), golden rain (Laburnum anagyroides), sweet bay (Laurus nobilis), privets (Ligustrum), Magnolia, Prunus, castor-oil plant (Ricinus communis), elders (Sambucus), whitebeams (Sorbus), lilacs (Syringa) and many other deciduous trees and shrubs.
In Japan it also feeds on Phellodendron amurense (Amur cork tree), Quercus acutissima (sawtooth oak), Styrax japonicus (Japanese snowbell), Cinnamomum camphora (Camphor tree), as well as Ailanthus altissima.


PUPA: 27--30mm. Cylindrical, but tapering towards both ends. Pale brown. Formed in a coarse, elongate, pear-shaped, double, unsealed dirty-brown cocoon, which is wrapped in a leaf and attached to the nearest twig by a silken peduncle. These hang from the plant all winter. The overwintering stage. A source of 'wild' silk in its native China.

Eupelmidae: Anastatus bifasciatus (Geoffroy, 1785); Tachinidae: Compsilura concinnata (Meigen, 1824), Exorista larvarum (Linnaeus, 1758), Exorista sorbillans (Wiedemann, 1830), Pales pavida (Meigen, 1824), Pales processioneae (Ratzeburg, 1840), Pales pumicata (Meigen, 1824).
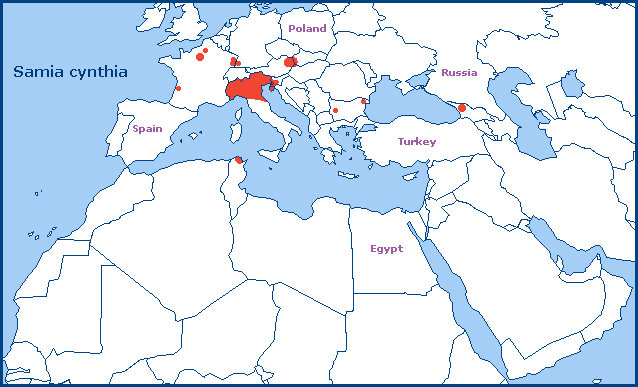
An introduced species found in towns and valleys where Ailanthus trees have become established, such as Paris, the Oise and Gironde (France), Alsace (France), southern Switzerland (Ticino/Tessin), northern Italy (Trentino Alto Adige, Garda, Bagnacavallo etc. (Sala & Bettini, 2005; Parenzan & Porcelli, 2006; Nardelli, 2019); Pietro Beretta, iNaturalist 2024), central Italy (Cattolica, Fiorenzuola di Focara, Pesaro, Fano (Lionello Gabucci, pers. comm. 2012)), northeastern Austria and Vienna, Hungary, the Istrian Peninsula (Croatia), Slovakia (Andrej Dobrovic, iNaturalist 2024), and central Slovenia. The population originally introduced into northeastern Spain (Barcelona) has now died out, and it appears to declining rapidly in northern Italy (Nardelli, 2019).
An isolated population is also present in the western Republic of Georgia (Zolotuhin, Didmanidze & Petrov, 2011; Christian Langner, iNaturalist 2024). Confirmed localities are Martvili, Batumi, Nokalakevi, Chokhatauri, Kharagauli, Ozurgeti, Marelisi, Gulripshi, Jikhanjuri, Khabume and Baghdati.
This species was introduced into Europe around 1856 in a bid to save the silk industry that was threatened by pébrine (pepper) disease, which effectively terminated sericulture using Bombyx mori at that time.
Subsp. ricini (Boisduval, 1854) has been released in several areas of Europe and appears to have become established in some, as have Samia 'canningi'-like populations (e.g. Slovakia). The latter may be 'hybrids' between ssp. cynthia and ssp. ricini.
Extra-limital range. This subspecies is endemic to the southern Russian Far East, northeastern China as far south as Zhejiang, and the Korean Peninsula. It has also been introduced into the eastern USA (in 1861), where it is found along the Atlantic coast from Connecticut to Georgia and west to northern Kentucky (Steve Walter, iNaturalist 2001). Other introduced population are present in Japan (Sado Island, Ishikawa, Aichi, Fukui, Toyama, Kanagawa, Chiba, Gifu, Miyagi and Niigata), India, Thailand, Canada, Venezuela, Uruguay and Brazil. Isolated populations may also be present in New South Wales (first recorded in Sydney in 1907) and Tasmania, Australia, and North Island, New Zealand (Baker, 1985); however, it is not known how stable these are.
As this species is reared by enthusiasts, and because many attempts have been made worldwide to establish local silk industries, individuals of Samia cynthia and its subspecies/races can often be found in many temperate and cool tropical regions.
Two, namely ricini (Boisduval, 1854) and pryeri (Butler, 1878); however, some taxonomists regard these as distinct species.
 Return to species list
Return to species list