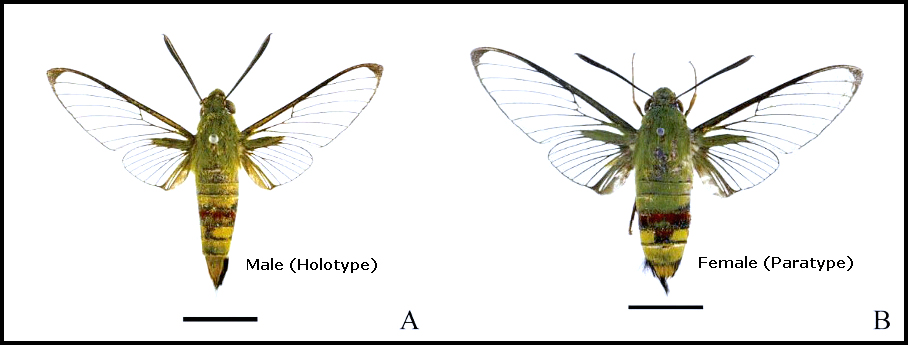
Cephonodes sanshaensis Deng & Huang, 2024, Journal of Asia-Pacific Biodiversity 17(1): 118. Type locality: China, Hainan Province, Xisha Islands [Paracel Islands/Hoŕng Sa Archipelago], Jinqin Island [Drummond Island], 26.vii.2021.
Note. Cephonodes sanshaensis differs from Cephonodes hylas (Linnaeus, 1771) in having an apical claw on the foretibia; Cephonodes hylas lacks a pronounced apical claw. Cephonodes sanshaensis is similar to Cephonodes kingii, but is immediately distinguishable by a smaller forewing apical patch. Among the known species of Cephonodes, this new species is visually most similar to Cephonodes picus (Cramer, 1777), but can be distinguished by the following characters: i) the right lobe of the uncus is hook-shaped with a distinctly acute apex, whereas in Cephonodes picus it is slender and rod-like; ii) the left valva is long and narrow with a truncate apex, whereas in Cephonodes picus it is broad and somewhat dilated apically; iii) the costa of the right valva is straight, but in Cephonodes picus it is slightly concave; and iv) the papillae anales in the female genitalia is small and suborbicular, but in Cephonodes picus it has a sharp tip.
Based on cytochrome-c oxidase I gene sequences (DNA barcodes), Deng, Wang, Tang, Cai, Ma, Wang & Huang, 2024, placed Cephonodes sanshaensis closer to Cephonodes xanthus Rothschild & Jordan, 1903 from southern Japan, with a genetic distance of 2.4%. However, unbeknownst to them, the holotype of Cephonodes sanshaensis falls into BIN: BOLD ACE8425, which also includes a sample from SW India and one from the Maldive Islands. With a genetic divergence of 1.39%, this BIN is actually closest to BIN: BOLD ABZ7280, which contains three samples from SW India currently identified as Cephonodes picus. Unfortunately, none of the samples from this latter BIN was included in the Deng et al. study.
Although Cephonodes sanshaensis does appear to be a good species, its relationship to Cephonodes picus needs further work. The type locality of C. picus is SW India; however, individuals from this area separate into the two DNA barcode BINs mentioned above, namely BOLD ACE8425 and BOLD ABZ7280. The lectotype of C. picus is a female in the NHMUK and so this needs to be DNA barcoded so as to allocate it to a BIN. In addition, additional females from SW India need to be dissected to see if there are two genitalial forms that can be identified there and associated with the BINs. To complicate matters, in the NHMUK there is a supposed male specimen of C. picus from Chiang Mai, Thailand. In the genitalial preparation [STI] the right valve looks different to all others depicted, but will have been flattened for photography and so is showing a different shape. This suggests that angle of view and degree of flattening can have a major effect on perceived shape, and so deductions drawn from genitalial preparations need to be treated with caution. In conclusion, the type of C. picus needs to be confirmed and fixed so as to affix the name to a BIN and a set (female) genital morphology. The genital morphology of the two SW Indian BINs (both males and females) need to be checked to see if they differ, and how they then relate to the lectotype of C. picus, the Thai male on the STI, and Cephonodes sanshaensis (Ian Kitching, pers. comm. 2024).
Note. The original date of publication for this species is 2024, not 2023 as is often quoted. The '2023' version (in both an online and PDF form) states '© 2023 National Science Museum of Korea (NSMK) and Korea National Arboretum (KNA), Publishing Services by Elsevier.' In the PDF form this statement is at the bottom of the abstract, with the following in the 'Article Info' - 'Available online 15 September 2023'. The online non-PDF form has the same 2023 copyright statement at the end of the paper. However, as in the PDF form, it also states - 'Received 27 April 2023, Revised 23 July 2023, Accepted 6 August 2023, Available online 15 September 2023, Version of Record 20 February 2024' (see under 'Show more' below the authors). In fact, as both forms have the same DOI they are both one and the same publication.
Both forms state quite clearly that the 'paper' was published on the 1 March 2024 in its final form in the 'Journal of Asia-Pacific Biodiversity, 17(1)', but with both having the ambiguous statement 'Available online 15 September 2023'.
We cannot find any 2023 version/form online which was published in 2023. We therefore have no idea if the 2023 version differs from what was finally published. Unless an actual 2023 version can be found online which has no reference to 2024, the publication date is 2024. There may have been a 2023 online version but what matters is that the date of the 'Version of Record' is explicitly stated to be 20 February 2024. The explicit definition of 'Version of Record' is given on the publishers website as 'The date the finalized version of the article was made available in the journal.' So even if a 2023 version does come to light, it cannot be taken as the 'Version of Record' as this is explicitly stated by the publisher to be the 2024 version. So we have concluded that the date of publication is unequivocally the 1 March 2024.
Wingspan 48-50mm in the male. Head covered with green hairs; compound eyes brown, with white hairs around the eyes; antennae clubbed, brown, apically hooked. Face white, proboscis brown. Thoracic tergites green. Foretibia with an apical claw. Forewing upperside with marginal band 2-2.5mm broad at Rs4, inner edge even between the veins. Upperside of abdomen green with a black and dark red belt; tergite VI with a black median patch, usually mixed with some red scales; the above colour pattern forms a funnel-shaped stripe. Underside of abdomen white, an oblique black and dark red band on each side of sternites I-IV extended to the end and connected in the centre. Upper side of anal tuft yellow-green with black hairs laterally, but underside black with white hairs centrally (Deng, Wang, Tang, Cai, Ma, Wang & Huang, 2024).
In the male genitalia, uncus quite asymmetrical, slightly tapering to apex, divided into two lobes by a medial groove, the left lobe a little longer than the right. Left lobe rectangular, each side parallel, apex with two small spines; right lobe strongly developed, forming a long, acute pointed hook; gnathos without processes, represented by a low ridge. Valvae asymmetrical, left valva long and narrow, each side almost parallel, with a truncate apex; right valva broad knife-shaped, costa straight with an arcuate ventral margin, cucullus blunt; juxta conical. Phallus slender, caudal 2/3 well sclerotized and tapering (Deng, Wang, Tang, Cai, Ma, Wang & Huang, 2024).
In the female genitalia, the suborbicular papillae anales is small and beset with scattered scales. Apophyses posteriores large, rod-like, with a rounded apex, and much longer than the apophyses anteriores, which has a somewhat club-shaped apex. Ductus bursae large, gradually broadening; corpus bursae small, bag-like, without signum (Deng, Wang, Tang, Cai, Ma, Wang & Huang, 2024).
The holotype was collected resting on a leaf of a species of Terminalia on the coast at 10m altitude, with the larvae found feeding nearby on Guettarda speciosa L. (Rubiaceae) (Deng, Wang, Tang, Cai, Ma, Wang & Huang, 2024).
China: 26.vii (Xisha Islands/Paracel Islands/Hoŕng Sa Archipelago).
OVUM: Unknown.
LARVA: Undescribed.
PUPA: Unknown.
Larval hostplants. Guettarda speciosa, a small tree (Deng, Wang, Tang, Cai, Ma, Wang & Huang, 2024).
Unknown.
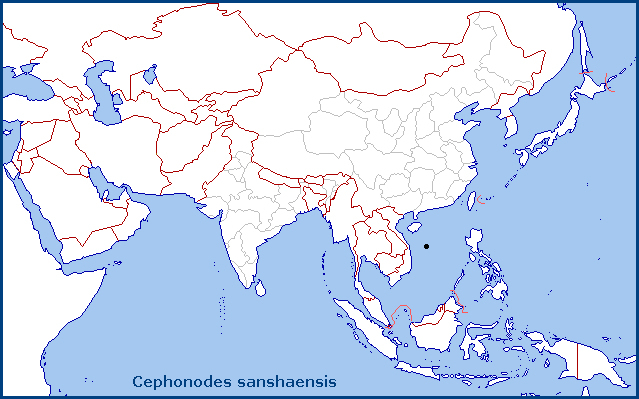
China: Hainan (Xisha Islands/Paracel Islands/Hoŕng Sa Archipelago).
So far, only known from Jinqin Island (formally Drummond Island) in the Xisha Islands/Paracel Islands/Hoŕng Sa Archipelago, South China Sea, China.
[However, an initial analysis and interpretation of specimens in the NHMUK and on BOLD, indicate that Cephonodes sanshaensis occurs from the Maldive Islands and SW India (maybe also Sri Lanka) eastwards across the Andaman Islands and the SE Asian mainland (Thailand, Cambodia, Vietnam) to the Xisha Islands/Paracel Islands/Hoŕng Sa Archipelago. Cephonodes picus (sensu BIN ABZ7280) seems to be restricted to SW India and perhaps Sri Lanka, with Cephonodes cunninghami (Walker, 1856) being the replacement species on the Greater Sunda Islands (Indonesia) and the Philippines eastwards to Queensland, Australia, and Tuvalu.]
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list