Type species: Sphinx anceus Stoll, 1781.
A genus containing eight to ten species from the Oriental, Australasian and eastern Palaearctic regions. One penetrates the western Palaearctic.
IMAGO: Upperside of wings predominantly brown and grey, the markings forming a tessellated pattern; outer margin of forewing serrate, sinuate between veins R4 and R5. Antennae rod-like, with terminal segment very long, filiform and rough-scaled. No eyelashes. Labial palpus large, rounded in side-view. Spines of abdomen numerous, the short ones pale, rather weak, the long ones stronger. Eighth tergite deeply sinuate, separate from sternite. Hindtibia with two pairs of spurs, the proximal spur twice as long as the distal.
Genitalia. In male, uncus simple, long, slender, slightly curved; gnathos shorter, broader, somewhat boat-shaped, with apex always sinuate. Valva large, sole-shaped, with three or four rows of large friction scales. Sacculus dilated at end, the dilated part armed with spine-like teeth which are directed upwards. Phallus with a dentate lobe on left side, continuous with a slender, acute process at right side. In the female, lamella postvaginalis suddenly narrowed distally; ostium bursae transverse, postmedian, sometimes covered with a bilobate ridge.
OVUM: Ovoid, smooth, shiny green.
LARVA: Head small, body tapering forward from the first abdominal segment, thoracic segment 3 and abdominal segment 1 with ventro-lateral flanges; horn of medium length, erect but sharply curved. Dorso-lateral longitudinal line present. Thoracic segments dark ventrally.
PUPA: Proboscis fused with body; abdominal segment 7 deeply undercut around its posterior margin; cremaster thick and short with a pair of apical spines, or extended into a shaft (Acosmeryx naga Moore, [1858]).
HOSTPLANT FAMILIES: Climbers and ramblers of the Vitaceae, Actinidiaceae and Dilleniaceae.
UK: Naga Hawkmoth, RUS: Gissarskii vinogradnyi Brazhnik
Philampelus naga Moore, [1858], in Horsfield & Moore, Cat. lepid. Insects Mus. Hon. East-India Company 1: 271.Type locality: [India, West Bengal,] Darjeeling.
(Taxonomic notes. (i) The subspecies Acosmeryx naga hissarica was based on specimens from the Gissar Mountains in Tajikistan and characterized by their generally smaller size and paler coloration. However, more extensive collecting in this country, as well as in Afghanistan and northern Pakistan, has revealed a range of colour intensity, from very pale moths to dark specimens that are indistinguishable from those from the eastern Himalaya and SE Asia. Thus, this would appear to be another example of the cline in colour intensity that was noted by Kitching & Cadiou (2000), in which sphingid species that occur along the southern edge of the Himalaya begin in the northwest as relatively small and pale moths and become gradually larger and darker as one moves east. Furthermore, DNA barcode analysis in BOLD clusters all samples of Acosmeryx naga from Tajikistan with Vietnam in a single BIN, ACF4345. Consequently, there is no justification for retaining subspecies status for Acosmeryx naga hissarica (Ian Kitching/Sphingidae Taxonomic Inventory, pers. comm. 2020).
(ii) DNA barcode data in BOLD does, however, support the division of Acosmeryx naga into two subspecies, represented by BINs ACF4345 and AAB5314. Acosmeryx naga naga comprises all populations from Uzbekistan and Tajikistan to Thailand and Vietnam (and so includes pale moths formally assigned to Acosmeryx naga hissarica). The second, namely Acosmeryx naga metanaga Butler, 1879, comprises the populations from central, eastern and northeastern China, Taiwan, Korea and the Russian Far East (and so includes Acosmeryx naga naganana of Zolotuhin & Yevdoshenko (2019)). Consistent morphological differences between the two subspecies are difficult to determine but, in general, Acosmeryx naga metanaga is smaller and has a less contrasted forewing upperside pattern (Ian Kitching/Sphingidae Taxonomic Inventory, pers. comm. 2020). The outer margin of the forewing is generally less crenulate too, although this character may be under some environmental influence as specimens from higher and colder localities tend to be more crenulate (Jean Haxaire, pers. comm. 2020).
(iii) Zolotuhin & Yevdoshenko (2019) (STI 22079) described Acosmeryx naga naganana based on a holotype and five paratypes from Taiwan (Miaoli County, 21km E Tungshih [Dongshi]). Despite the name, Acosmeryx naga naganana was said to be a large subspecies. In the description it was said to differ from the nominotypical subspecies by the smooth external forewing margin. However, this character may be under some environmental influence as specimens from higher and colder localities tend to be more crenulate (Jean Haxaire, pers. comm. 2020) and so is not useful for distinguishing subspecies. Acosmeryx naga naganana was distinguished from the Japanese Acosmeryx naga metanaga by the absence of a brownish or reddish tint to the ground colour, the presence of distinct dark triangles on the abdominal upperside, an S-shaped (rather than straight) pale marginal band on the forewing upperside, and an 'almost straight not angled in costal zone upper part of dark postmedial notch'. However, examination of the long series of Acosmeryx naga from Taiwan and Japan in the NHMUK has shown that none of these characters is consistent. With regard to the male genitalia, Zolotuhin & Yevdoshenko (2019) (STI 22079) characterized those of Acosmeryx naga naganana as 'valvae egg-shaped (ovoid) not parallel-sided as in most other subspecies, valvar appendix [harpe] more compact and regularly dentate on the inner surface, phallus a bit shorter but with distinct apical spur which is strongly curved and lance-shaped'. Zolotuhin & Yevdoshenko (2019) (STI 22079) illustrated only the male genitalia and phallus of the holotype of Acosmeryx naga naganana and a specimen from Kunashir (Kurile Islands), together with just the phallus of a specimen from North India. The purported diagnostic features of the valve shape are inconsistent in that the shapes are different between the left and right, which is probably due to distortion when the genitalia were flattened. The valves of the holotype of Acosmeryx naga naganana are indeed more rounded than the left valve of the Kunashir specimen, but less rounded than its right valve. The shapes of the harpe are well within the range of individual variation of Acosmeryx naga and the reason that the apical transverse process of the phallus appears to have a diagnostic 'strongly curved and lance-shaped apical spur' is that this process has been lost from the other two illustrated preparations. Furthermore, the minimum DNA barcode divergence between samples from Japan and Taiwan is only 1.082%, less than the 1.186% maximum divergence within Acosmeryx naga naga (excluding the Thailand and Vietnam samples), and all samples of Acosmeryx naga from Japan, Taiwan and mainland China cluster in a single BIN, AAB5314. Consequently, there is no justification for retaining Acosmeryx naga naganana as a subspecies separate from Acosmeryx naga metanaga (Ian Kitching/Sphingidae Taxonomic Inventory, pers. comm. 2020).)
Palaeotropical; Oriental region. Sindian refuge.


Wingspan: 80--112mm. Apart from Acosmeryx purus Kudo, Nakao & Kitching, 2014, the most conspicuously marked species of the genus, easily distinguished by the pattern of its forewing. Forewing upperside with a brown discal band extending from the costa towards the middle of the distal margin, sharply defined anteriorly, the triangular area anterior to it grey; a well-defined, grey submarginal band running more or less straight from vein Rs4 to the tip of 1A+2A.
In the male genitalia, gnathos parallel-sided. Harpe with process quite acute distally, resembling a hand with the thumb lying alongside the forefinger, with the other fingers curved back and upwards. Phallus with left lobe shorter than in all other species of Acosmeryx. In the female genitalia, ostial plate similar to that in Acosmeryx anceus anceus.


Occurs in cultivated as well as uncultivated mountain valleys and ravines. Along the southern slopes of the Gissar Mountains of Tajikistan (Shchetkin, 1956) it is confined to damp locations in gorges at 1100--1700m which support wild stands of Juglans regia (walnut) and Acer turkestanicum (Turkestan maple) overgrown with wild Vitis vinifera (grape vines). In Afghanistan it occurs at 1500--2500m (Daniel, 1971).
Bivoltine; late April to June, and again in late July (Ebert, 1969; Daniel, 1971; Derzhavets, 1984).
OVUM: Size unrecorded; almost spherical, smooth, a deep rich green, becoming whitish before hatching (Bell & Scott, 1937).
LARVA: Full-fed 70-100mm. Dimorphic: green or brown.

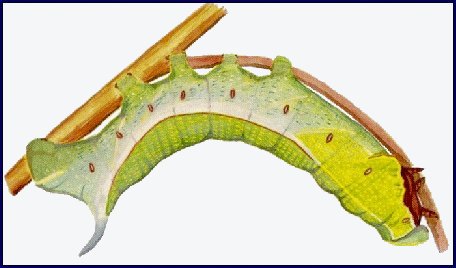
According to Bell & Scott, 1937, in the first instar, the head and body are green, the horn black, long and straight. In the second instar, thoracic segment 1 is as narrow as the head, with the body tapering sharply anteriorly from the first abdominal segment. The black horn is still long and straight, but reddish at the base and with a white tip. The head and anal segments are yellow, the body green dotted with white, with the spiracle of abdominal segment 1 surrounded by a black spot. A yellow dorso-lateral stripe runs from the head to the horn. This colour pattern remains the same for the next two instars, but a lateral flange develops on the third thoracic and first abdominal segments and a yellow ventro-lateral stripe appears on the thoracic segments and abdominal segment 1. In the final instar of the green form, the head is grass-green, with a narrow, pale yellowish dorso-lateral stripe and a broader stripe of the same colour separating face from cheek; thoracic segments 1 and 2 grass-green with short darker stripes; the rest of the body bluish green, mottled with yellow above the dorso-lateral stripe and pale greyish blue below. The dorso-lateral stripe is narrow and white on thoracic segments 1 and 2, broader and pale yellow on thoracic segment 3 and abdominal segment 1, then white to the base of the horn, and edged narrowly above with orange on segments 3--7. The narrow, white ventro-lateral stripe on thoracic segments 1 and 2 becomes broad and yellow as it outlines the flange before turning upwards on abdominal segment 1 to form an oblique stripe. There are also pale yellow, oblique lateral stripes on segments 1--7. The horn is lilac-grey dotted with purple. There are dark purple patches on the body above the bases of the legs, increasing in size caudally, and extending along the lower edge of the flange on thoracic segment 3 and abdominal segment 1. Upper part of prolegs bluish, the lower part pale yellow; feet brown, anal clasper bluish, anal flap edged broadly with pale yellow. Spiracles deep orange. In the brown form the green pigmentation is replaced by pinkish brown. The whole body is smooth and moderately shiny.
At rest on the underside of a leaf or stem, the larva throws back its head and anterior segments in a sharp curve, the head held so that the face is in the same plane as the dorsum of thoracic segment 1. The thoracic legs are pressed close to the body and the flange is dilated laterally. As in Clarina kotschyi (Kollar, [1849]), which it closely resembles, the alarmed larva withdraws the head and first two thoracic segments into segment 3; unlike Clarina kotschyi, thoracic segment 3 and abdominal segment 1 are puffed out and the flanges further dilated.
Occurs from June until September.
Major Hostplants. Vitis and Ampelopsis (both Vitaceae). On Vitis vinifera in Kabul, Afghanistan (Daniel, 1971).
Minor Hostplants. Actinidia and Saurauia (both Actinidiaceae).
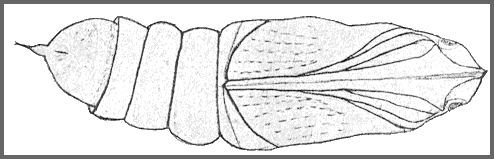
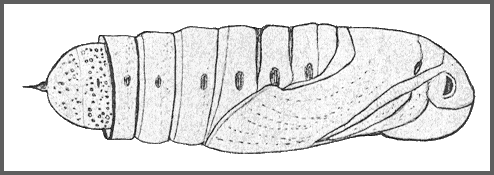
PUPA: 44--55mm. Very similar in shape to Clarina but dark brown with the hind margin of abdominal segment 7 deeply undercut. Between the frons and the proboscis, there is a knobbly pentagonal sclerite. Abdomen marked with dark pits and stripes. Cremaster small, basal half bulbous, distal half a cylindrical shaft ending in two small hooks. Formed in a slight cocoon on the ground. Very sensitive to desiccation (Shchetkin, 1956), highly tolerant of moisture, and very immobile. The overwintering stage.
The pupa overwinters partly formed up, so that much of the adult wing pattern can be seen through the wing cuticle.

None recorded.
Within the region, only the Gissar Mountains of southern Tajikistan (Shchetkin, 1949; Shchetkin, 1956; Derzhavets, 1984), southern Uzbekistan and Afghanistan (Wiltshire, 1961; Ebert, 1969; Daniel, 1971; C. M. Naumann, pers. comm.). Shchetkin (1956) believed this population to be an arcto-tertiary relict (along with numerous plants ) which developed in the western Tian Shan and which has since spread to Afghanistan, where it has come into contact with the nominate subspecies, which spread from the east. However, a more plausible explanation is that this population is of Pleistocene origins, having taken refuge in the Sindian refuge, from whence it spread as far northwestwards as the present-day climate would allow. This refuge has been important in the evolution of several Palaearctic sphingid populations, species and subspecies, most notably Hemaris rubra Hampson, [1893], Hyles nervosa (Rothschild & Jordan, 1903) and Deilephila rivularis (Boisduval, [1875]).
Extra-limital range. The Himalayan foothills of Pakistan, India and Nepal (Haruta, 1992) to Burma/Myanmar, Thailand, Laos, Vietnam (Sa Pa) and, maybe, Peninsular Malaysia.
As subsp. metanaga Butler, 1879, central, eastern and northeastern China (Bell & Scott, 1937) to Taiwan, Korea and Japan (D'Abrera, 1986). Several migrants have been reported from the Russian Far East since 2002-2003, as far northeast as Kunashir Island (Beljaev, 2003; Rybalkin & Yakovlev, 2017). It may even have established a resident population southeast of Ussuriisk (Dubatolov, pers. comm. 2013).
[It should be noted that some of the individuals of this subspecies recorded from southern China and northern Vietnam may, in fact, be the newly-described Acosmeryx purus Kudo, Nakao & Kitching, 2014.]
 Return to species list
Return to species list