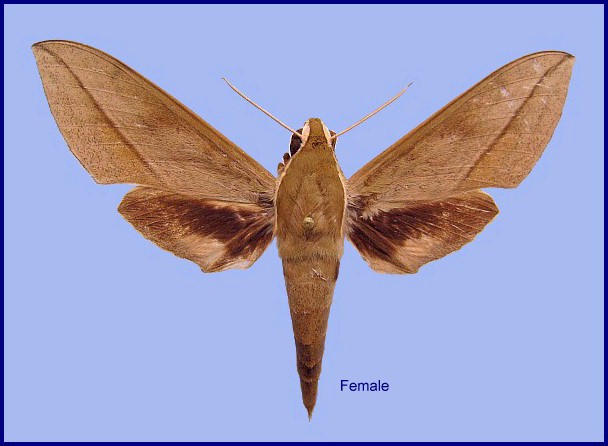



Theretra tibetiana Vaglia & Haxaire, 2010, In: Vaglia, Haxaire, Kitching & Liyous, 2010, European Entomologist 3(1): 21. Type locality: China, Xizang/Tibet, Motuo Nature Reserve, 28.viii.2006, 900m.
Note. The research of Vaglia, Haxaire, Kitching & Liyous, 2010 has demonstrated that the taxon known as 'Theretra clotho' (Drury, 1773) actually consists of two cryptic species, the more southern Theretra clotho, which just enters tropical southern China, and the more northern Theretra tibetiana, which occurs from Xizang/Tibet across central and eastern China to Taiwan and Japan. Unfortunately, they are visually almost impossible to tell apart, but both species can be clearly differentiated via DNA barcodes.
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan under Theretra clotho, as well as Moths of the southern Shikoku, Japan.]
Wingspan: 70--100mm. Head and thorax greenish/brownish-grey, with a white lateral stripe from palpus to rear of thorax. Abdomen also greenish/brownish-grey, with a black lateral patch near the thorax (second segment). Forewing uppersides greyish-brown with a sharp brown oblique postmedial line from inner margin to apex, and a fine black discal spot. Hindwings black basally, brown distally, with a buff patch at the anal angle. Antennae and legs white.
In other words, very similar to Theretra clotho (Drury, 1773); however, Theretra tibetiana can be distinguished by its more rounded shape, its darker colour (more slate grey), the generally thicker postmedial line, and the characteristic shape of its genitalia. On the forewing underside there are usually three postmedial lines rather the two as found in Theretra clotho. The background colour of the wing undersides is pinkish-brown or pinkish-grey (yellow, ocher or orange in Theretra clotho), and speckled with fine, dark spots (Vaglia & Haxaire, 2010). There are far fewer of the latter in Theretra clotho.


Frequents open forest, forest edge, orchards, plantations, wooded scrub, suburban gardens and city parks. A species which likes humid areas, being confined to regions with medium to heavy rainfall.

China: vi-vii (Guizhou; Jiangxi; Sichuan); vi-viii (Shanghai; Zheijiang); vii-viii (Xizang/Tibet); ix (Zhejiang). Taiwan: iv-ix (Hualien Hsien); v (Pingtung Hsien); vii (Pingtung Hsien); ix (Pingtung Hsien). Japan: 23.iii (Ryukyu Archipelago); 2.vi (Shikoku); 16.vi-14.viii (Honshu; Kyushu; Tsushima; Ryukyu Archipelago).
In Shanghai, China, moths are usually only met with in June, July and August (Mell, 1935). Kendrick (2002) states that this species (and/or Theretra clotho) is multivoltine in Hong Kong, occurring from April until late October, with peaks in mid April, late May, mid August, and mid October.
Park et al. (1999) give August as the flight period in Korea.
OVUM: [Unknown, but this description by Bell & Scott (1937) for Theretra clotho may also apply to this species.] Pale green, changing to yellowish green just before hatching. Slightly oval (1.8 x 2.0mm), shiny and finely pitted.
LARVA: [Unknown, but this description by Bell & Scott (1937) for Theretra clotho may also apply to this species.] Full-fed 75--85mm, width 12mm, horn 9mm. In the first instar honey-yellow, but green after feeding; horn black, long, straight, minutely bifid. In the second instar, head and body yellowish-green. There is a narrow yellow dorso-lateral stripe, with a black eye-spot, ringed with white, on segment 5. By the third instar, head and body pale green, with short, dark green lines. A dark green dorsal stripe is present, as is a pale dorso-lateral stripe from segment 6 to base of horn. There is an eye-spot on segment 5, which is enamel-white with a large black pupil, in the middle of which is a longitudinally elongate-oval blue spot, the whole being edged narrowly with dark green above, black below. A much smaller eye-spot occurs on 6 and further eye-spots of decreasing size on 7 to 11; these are white edged above with black. Horn, long, straight, black with base orange except for a brown dorsal spot; end red with the extreme tip white. In the fourth instar, head green, body grass-green dotted with pale yellow; dorso-lateral stripe yellow; eye-spot on 5 yellow with green pupil, in which there is a horizontal blue line, the whole edged narrowly with black. Horn long, thin, minutely bifid, slightly up-curved, base blood-red, then fuscous shading to black, tip yellow or pink. The ground colour varies a good deal and there may be blind eye-spots on segments 6 to 11.
In the fifth and final instar, head slightly shiny, covered sparsely with minute rounded tubercles. Clypeus one-half length of head, apex very acute, basal angles slightly rounded; apex of false clypeus forming an arch over that of true clypeus; labrum one-third as long as and as broad as clypeus, longitudinally corrugate; ligula longer than labrum, elongate kidney-shaped; cutting-edge of mandible slightly waved. Body dull and smooth, shaped as in others of the genus. Horn of medium length, sharply down-curved, stout at base, tapering gently to near the tip where it narrows sharply to a point; surface dull, covered sparsely with small tubercles.
In the green form, head glaucous-green; labrum and ligula honey-yellow; basal segment of antenna green, other segments rusty; mandible green with the tip reddish-brown; eye 1 whitish, the rest black. Body glaucous-green or yellowish-green, speckled with darker green on segments 6 to 11. There is a narrow interrupted dark green dorsal stripe from 6 to base of horn, and a large dorso-lateral shortly-oval eye-spot on 5. The pupil of this is dark green, with a pale blue stripe, edged with darker blue, running horizontally across it. The pupil itself is edged with a broad ring of primrose-yellow, at the lower edge of which is a narrow crescent of crimson; the whole edged narrowly with dark green or black. There are small dorso-lateral, longitudinally oval, pale yellow blind eye-spots or dots (edged above with green) on 6 to 11, but these are sometimes missing, sometimes on 6 only. Running between these is an obscure whitish or yellowish dorso-lateral stripe, broken by the yellow spots, from 6 to base of horn. Horn pale purple; true legs purple with a yellow band on the basal segment. Spiracles pale earth-colour, edged in front and behind for half their length with rose-colour or purple.
In the rarer dark form the green body-colour is replaced by dull brown, and there are dark brown oblique patches on abdominal segments 3-8.
In full-fed larvae the anterior segments are not so strongly retractile as in others of the genus, and when in the resting position the anterior segments are more contracted ventrally than dorsally the head being held with the face directed downwards. The larva has the peculiar power of inflating the thoracic eye-spots at will, so that they become quite prominently convex.
Larvae tend to be found on vines cascading over walls or hanging down from trees. At night they often crawl to the end of shoots, where they can be easily seen. By day they conceal themselves deep in the foliage, resting with the head tucked up under the thorax. Between 25 June and 2 July 1995, numerous full-grown larvae were found on Parthenocissus growing over walls in Hangzhou, Zhejiang, China (Pittaway & Kitching, 2000).

PUPA: [Unknown, but this description by Bell & Scott (1937) for Theretra clotho may also apply to this species.] 60 mm, width 14 mm. Head, thorax and wing-case soiled brown, with mottling of a much paler shade, except on tongue-case. Abdomen of a bone-colour, suffused and lined with soiled brown except on dorsum and round the spiracles. There is a broad, ill-defined, greenish dorsal stripe; a round, pale yellow subdorsal spot near the front margins of 6 to 10; hind, bevels of 8 to 10 dull yellow speckled with black, front bevels of 9 to 11 pink mottled with brown; spiracles black; cremaster with basal half yellowish, distal half black.
Head subquadrate, shoulders rather prominent, tongue-case not much projecting frontad (3 mm) but projecting considerably ventrad; nearly semicircular in side-view, with a narrow mesial channel. Antenna slightly longer than fore leg, which reaches to about the middle of the wing-case, mid-leg to two-thirds the distance to the tip; there is a narrow coxal piece. Surface dull and smooth, with a deep palpal depression at edge of tongue-case below eye. Front bevel of segment 9 with four or five narrow, more or less parallel, ante-spiracular ridges; similar but fewer ridges on 10 and 11. Spiracle of 2 a narrow slit lying between the slightly raised hind margin of 2 and a transverse, rounded-oblong, depressed area running back from the front margin of 3, the whole lying at the bottom of a deep depression; remaining spiracles elongate-oval, central slit with thick rim. Cremaster triangular, broad and deep at base, ending in two slightly diverging conical teeth, their bases touching, each tooth minutely bifid at tip; upper surface convex and coarsely shagreened, lower surface concave; under base of cremaster a wide, deep funnel-shaped depression runs axially forward into 14.
Formed in a flimsy, brown cocoon amongst debris on the ground. The pupa is lightly attached to one end of the cocoon by the points of the cremaster.
Larval hostplants. Recorded in Hangzhou, Zhejiang, on Parthenocissus (Pittaway & Kitching, 2000). Chu & Wang (1980) also list Cissus for China. May also feed on other Vitaceae as well as various Actinidiaceae, Araceae, Begoniaceae and Dilleniaceae, as per Theretra clotho.
Recorded in Taiwan from Alocasia odora, Ampelopsis brevipedunculata, Ampelopsis cantoniensis, Causonis japonica [syn. Cayratia japonica], Impatiens walleriana, Leea guineensis, Parthenocissus tricuspidata, Saurauia tristyla, Tetrastigma formosanum and Vitis heyneana [syn. Vitis kelungensis].
Recorded in Japan from Ampelopsis brevipedunculata (Nozakai & Miyata, 1989) and Actinidia rufa (Nakajima, 2013).
Unknown for China.
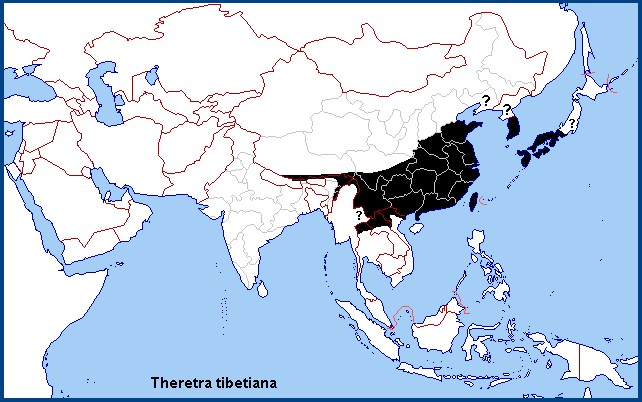
China: Anhui (Mt. Huang Shan); Shanghai; Zhejiang (Tianmu Shan; Kuocang Mountain Nature Reserve); Hubei (Lichuan); Sichuan (Chongqing; Qingchuan); Xizang/Tibet (Mutu, Namjagbarwa area, 850m; Motuo Nature Reserve, near Motuo/Metok/Mędog/Pemako); Guizhou (Leishan, Leigongshan, 1300m); Hunan (Dayong); Jiangxi (Wuyi Shan); Fujian (Longqi Shan; Guangze, 1200m); Guangdong (Guangzhou); Hong Kong (Roger Kendrick, pers. comm. 2010).
Some Chinese records from Xizang/Tibet may be suspect as the specimen illustrated by Zhang et al. (1986) is Theretra boisduvalii (Bugnion, 1839).
Taiwan: Hualien Hsien (Taroko National Park); Taipei (Yangmingshan); Taipei Hsien (Fushan; Wulai); Chiayi Hsien (Chiayi; Shanmai, 800m); Tainan Hsien (Kuantzuling); Taichung; Nantou Hsien (Jenai; Puli); Kaohsiung Hsien (Shanping; Liukuei); Pingtung Hsien (Kenting).
South Korea: South Cholla Province (Geumo-do); South Kyongsang Province (Jishim-do; Geoje-san).
Japan: Honshu (Kobe; Mt. Oboshiyama; Yamaguti); Shikoku (Usa); Kyushu (Naze; Taken; Yuwan); Tsushima (Sasuna); Ryukyu Archipelago (Iriomote-jima; Okinawa; Yaku-jima).
From Xizang/Tibet east through Bhutan (Irungbam & Irungbam, 2019) and southern China to eastern China, Taiwan, South Korea and Japan, and south to central Vietnam (Le & Vu, 2024), northern Laos and northern Thailand. Also, south to Manipur, India (Jatishwor Irungbam & Fric, 2021). An uncommon migrant to northern China.
Oriental.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list