

Macroglossa caudata Bremer & Grey, 1853, in Motschulsky (ed.), Etudes ent. 1: 62. Type locality: [China,] Pekin [Beijing].
Synonym. Macroglossa caudata Bremer & Grey, 1853.
Synonym. Sphecodina caudata angulifascia (Mell, 1922).
Synonym. Sphecodina caudata meridionalis Mell, 1922.
Synonym. Sphecodina caudata angulilimbata Clark, 1923.
Note. The population in northern Thailand has been split off and described as a new species, namely Sphecodina antonkozlovi Haxaire, 2021, this being based based on a single male collected in March 1988 at a fruit trap on Doi Inthanon, Chiang Mai Province. This population is very isolated from the main populations of Sphecodina caudata farther north in China. It is separated from Sphecodina caudata by its larger size, darker pattern and genital structure (Haxaire, 2021).
Wingspan: 62--67mm. Distal margin of forewing even, slightly concave anteriorly and posteriorly, neither deep bisinuate nor lobed. Abdomen more broadly tufted at end than in the North American Sphecodina abbotti (Swainson, 1821).



A diurnal species in the Russian Far East, flying mainly in the morning (08.00-10.00am) around meadows in mixed coniferous/deciduous and deciduous woodland, particularly those characterized by Quercus mongolica (Izerskiy, 1999b; Dubatolov, pers. comm. 2010). Frequents the flowers of Rhododendron schlippenbachii Maxim., although males are more attracted to excrement (Koshkin, 2013), urine (Koshkin & Bezborodov, 2019), fermenting/ripe fruit (Haxaire, 2021), and suger-rich tree exudates (see photos below). Hovering swarms of males can easily be mistaken for hornets.
This species is long lived and may only mate after several days, with mating taking 2-3 hours. Thereafter, oviposition is a long, drawn out affair, with 1-2 ova being deposited per female per night (in captivity) (Mark O'Neill, pers. comm. 2023).




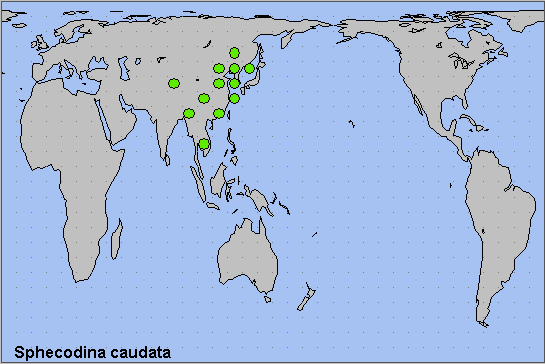
China: i-iii (Guangdong); 12.iii (Fujian); iv (Hubei); v (Chongqing; Shandong; Beijing); vi (Zhejiang; Henan); vi-vii (Guangdong; Guizhou; Zhejiang); viii (Guangdong). Russia: 12.v-vi (Primorskiy Krai); 29.v-10.vii (Khabarovskiy Krai); 10.vi (Yevreyskaya); 19.vii (Primorskiy Krai).
An early species which Mell (1935) recorded as having two generations in Guangdong.
Park et al. (1999) give June and July as the flight period in Korea.
OVUM: Greenish-yellow, turning to a cream colour just before they hatch. Surprisingly large for the size of moth (2-3mm in diameter) and laid on the the young flowering shoots and underside of leaves of the host (Mark O'Neill, pers. comm. 2023).

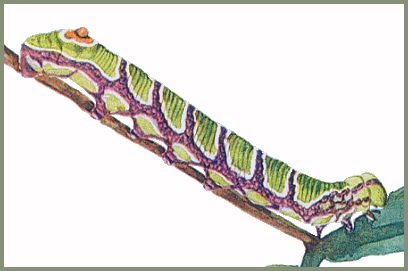
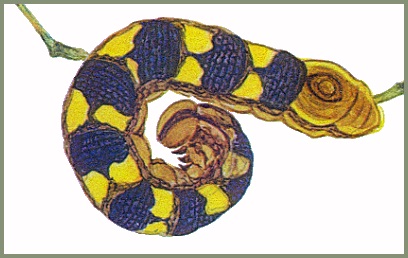
LARVA: Full-fed 39--42mm. Has two main colour forms - green and brown, and grey. Newly hatched L1 larva 8mm, cream-coloured, with a 3-4mm black horn and white head (Mark O'Neill, pers. comm. 2023). By the third instar the pale larvae resemble sawflies and will curl up into a spiral if disturbed; larger larvae will do the same. When ready to pupate larvae undergo a dramatic colour change, becoming brown with large orange patches down the side. When wandering on the ground they respond to threats in an equally dramatic way. Curling into a spiral they raise the anal segments. Viewed laterally, this creates a very good impression of a small viper. [All stages are illustrated in Koshkin, 2023]
This species shows a preference for clumps of Parthenocissus growing over large bolders, walls and rockfaces, usually within woodland clearings or along woodland edges (Pittaway, pers obs. 2016).










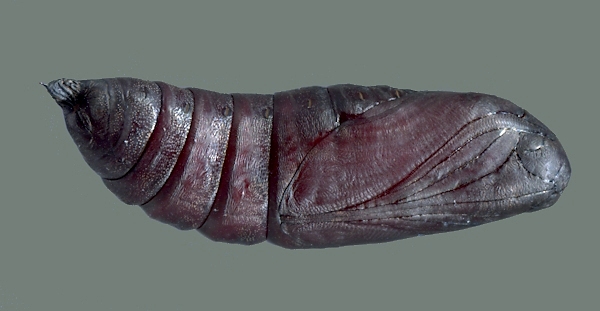
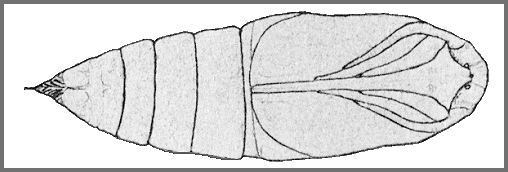
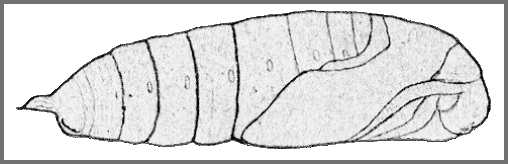
PUPA: 43--45mm.
[Illustrated in Koshkin, 2023]


Larval hostplants. Vitis amurensis in the Russian Far East (Izerskiy, 1999b; Anton Kozlov, pers. comm. 2011; Koshkin, 2013). In China, usually on Parthenocissus tricuspidata, Ampelopsis brevipedunculata and the introduced Parthenocissus quinquefolia growing over bolders and up walls and cliffs (Pittaway, pers. obs. 2004, 2016). These hosts are also utilized in the Russian Far East, as are commercial grapes (Koshkin, 2013).



China: Beijing (Baihua Shan; Haidian; Huairou District; Qianlabagou); Shandong (Qingdao; Weihai; Yantai; Jinan); Henan (Sanmenxia; Zhengzhou); Anhui (Mt. Huang Shan); Zhejiang (Tianmu Shan; Hangzhou; Quzhou; Lishui; Taizhou); Hubei (Wuhan); Sichuan (Xiling Xueshan National Park; Ya'an; Mengding Shan); Chongqing (Simian Mountain National Scenic Resort); Yunnan (Yanmen; Mingshan, nr. Ya'an); Guizhou (Guiyang; Qiannan Buyei and Miao Autonomous Prefecture; Qiandongnan Miao and Dong Autonomous Prefecture); Hunan (Mangshan); Fujian (Fuzhou; Ningde; Xipu Reservoir; Jianning County); Guangdong (E. Nan Ling; Guangzhou; Shaoguan; Nanling National Forest Park; Ruyuan Yao Autonomous County).
North Korea: North Hamgyong Province (Jueul, 1500m).
South Korea: Kyonggi Province (Gwangleung); Kangwon Province (Hwacheon; Gyebang-san); South Cholla Province (Baekyang Temple); North Kyongsang Province (Juwang-san).
Russia: Amurskaya (Arkhara District, Khingan Nature Reserve, 3km W of Kundur; Blagoveshchensk); Yevreyskaya (Bastak Nature Reserve); Khabarovskiy Krai (Bolshekhekhtsyrskii Nature Reserve, Khabarovsk suburbs; Khabarovsk City; Bychikha; Durmin River, Lazo District; Vyazemsky District; Sysoevsky Utes); Primorskiy Krai (Anisimovka; Primorskiy; Rettikhovka: Barabash-Levada; Vladivostok; Narva; Kalinovka; Anuchino; near Kalinovka; Gornotayezhnoye; Brovnichi; Tigrovyi; Kedrovaya Pad' Nature Reserve; Lazovsky Nature Reserve; Andreevka; Golubinyi Utes; Pozhiga Village, Dalnerechensk District; Khasan).
The southern Russian Far East as far north as the Amur Oblast (Koshkin & Bezborodov, 2019; Koshkin, 2023; Koshkin & Kuzmin, 2023), the Korean Peninsula, eastern and southern China. Appears to be expanding northwards in the Russian Far East (Koshkin & Kuzmin, 2023).
An isolated population in northern Thailand, formally included within Sphecodina caudata, has been described as a new species (Haxaire, 2021).
A closely related species occurs under similar climatic conditions in eastern North America.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list