

Hyloicus pinastri arestus Jordan, 1931, Novit. zool. 36: 244. Type locality: [Russia, Khabarovskiy Krai,] Nikolajewsk [Nikolayevsk-na-Amure], mouth of Amur [river].
Synonym. Sphinx pinastri arestus (Jordan, 1931).
Synonym. Sphinx hakodoensis O. Bang-Haas, 1936, Ent. Z., Frankf. a.M. 50: 254. Type locality: Corea sept. [North Korea], Hakodo Province [North Hamgyong Province], Seishin [Chongjin], Siren Mountains, 250m; [North Korea,] Poktussan [Baekdu-san/Changbaishan].
Synonym. Sphinx laricis Rozkhov, 1972.
Synonym. Hyloicus morio heilongjiangensis Zhao & Zhang, 1992, Acta entomol. sin. 35: 95. Type locality: China, Heilongjiang, Dai Ling Mountains [Dailing, south of Yichun].
Note. Hyloicus morio is now recognized as a species distinct from the European Hyloicus pinastri (Linnaeus, 1758), with two subspecies. Subsp. morio Rothschild & Jordan, 1903 inhabits Hokkaido, and central and northern Honshu, Japan (Owada & Kogi, 1992); arestus Jordan, 1931 occurs from Korea, north-eastern China and the Russian Far East across southern Siberia and Mongolia to the Altai (Owada & Kogi, 1992) and southern Urals in european Russia.
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan.]
Wingspan: 60--80mm. Very similar to Hyloicus pinastri in external appearence, although many individuals are tinged with reddish-brown. However, the proboscis is only half as long as it is in Hyloicus pinastri (less than 1 cm vs. more than 2 cm in the latter). Even more similar to Hyloicus morio morio, the slight differences being: black stripe on tegula narrower, the stripe between it and the wing base duller grey; forewing upperside more uniformly grey, the brown shading on the hind margin less prominent; dark streak behind vein M1, itself faint in Hyloicus morio morio, absent or vestigial; apical line fainter.
In the male genitalia, the upper branch of the sacculus is approximately equal to the lower in length, slightly curved, flattened and broad. This is markedly different from that of Hyloicus pinastri, where the upper branch is long, curved and cylindrical. Phallus with apical groove shorter than in Hyloicus morio morio.


A species of cool larch (Larix) and pine (Pinus) forests.
According to Litvinchuk (1986), females of Hyloicus morio arestus call and mate only during the morning. This behaviour differs from that of Hyloicus pinastri and may explain how these two species became reproductively isolated. A similar mechanism isolates Hyles euphorbiae (Linnaeus, 1758) and Hyles tithymali (Boisduval, 1834) (Pittaway, 1995), Hyles euphorbiae and Hyles gallii (von Rottemburg, 1775) (Pittaway & Kitching, 2000) and Smerinthus kindermannii Lederer, 1853 and Smerinthus ocellata ocellata (Linnaeus, 1758) (Pittaway & Kitching, 2000).
China: 27.v-8.vi (Shaanxi); 16-18.vi (Heilongjiang); 9.viii (Liaoning). North Korea: v (North Hamgyong Province). South Korea: 19.iv (Jiri-san); 4.v (Soku-ri); 1.viii (Baekun-san). Japan: 30.iv (Hahajima). Russia: 16-30.vi (Siberia, Tomsk); 18.vi (Siberia, Chingisy); 26-27.vi (Khabarovskiy Krai); 4.vii (Siberia, Bazoy); 4-24.vii (Amurskaya; Primorskiy Krai); 10.vii-4.viii (Khabarovskiy Krai); 16.vii (Altai); 22-28.vii (Siberia, Chingisy); 24-25.vii (Khabarovskiy Krai); 26.vii-12.viii (Siberia, Novosibirsk); 6.vii-10.viii (Altai); 15.viii (Siberia, Tomsk).
Danner, Eitschberger & Surholt (1998) state that around Novosibirsk (Siberia, Russia) the main flight period is between 18.vi and 28.vii.
Park et al. (1999) give late April until early October as the flight period in Korea.
OVUM: Pale green at first, changing to reddish yellow. Oval (1.5 x 2.5mm), slightly dorso-ventrally flattened, shiny and smooth. Each female lays between 100 and 120 eggs, which hatch 13--15 days later (Zhao & Zhang, 1992).
LARVA: Full-fed 54--65mm. Dimorphic: predominantly green or brown. Similar in both appearence and behaviour to Hyloicus pinastri.
Mature larvae may be either dark leaf-green with a marked purplish-brown dorsal band bordered on either side with a greyish-white stripe, and two broken cream or white longitudinal lateral stripes (dorso-lateral and ventro-lateral), or mainly greyish-brown. In both colour forms the whole body, which is slender and of even thickness, and blends well with surrounding twigs, has dark, sunken, encircling lines (more evident than in Hyloicus pinastri), apparently dividing it up into narrow rings behind a large, prominent, ochreous-colored, black-lined head and shield. The body is smooth, but not glossy, unlike the head, underside, legs and shield. However, an oily appearance extends over the entire body as the larva darkens prior to pupation.
Mature larvae can easily be told apart from those of Hyloicus pinastri by the well defined dark, purple-brown, dorsal band with its greyish-white edging. This dorsal band is more diffuse and reddish-brown in Hyloicus pinastri, and not edged with greyish-white. The basic green body colour of H. morio arestus also has a more emerald hue than that of Hyloicus pinastri. However, both have a thin, slightly curved, black, knobbly horn.
Found mainly during July and August. [All stages are illustrated in Koshkin, 2023]

PUPA: 30--40mm. Very similar to that of Hyloicus pinastri, except that the free tongue-case is replaced by a small, blunt knob. [Illustrated in Koshkin, 2023]
Larval hostplants. Danner, Eitschberger & Surholt (1998) state that around Novosibirsk (Siberia, Russia) the main hosts are Pinus sibirica, Pinus sylvestris and species of Abies. Zolotarenko, Petrova & Shiryaev (1978) give Pinus sibirica, Larix sibirica and Larix gmelinii for Siberia. Around Lake Baikal (Siberia) the natural hosts are Larix sibirica and Pinus sibirica (Rozhkov, 1956). In Amurskaya, Russia, recorded from Larix gmelinii and Pinus sylvestris (Streltzov, Osipov & Malikova, 2003).
In the Altai (Russia), this species can be a major pest of Pinus sylvestris, sometimes causing complete defoliation of large areas (Plotnikov & Gninenko, 1980).
Recorded from Heilongjiang, China, (as Hyloicus morio heilongjiangensis) on Abies fabri, Larix gmelinii, Picea asperata, Pinus koraiensis and Pinus sylvestris var. mongolica (Xiao, 1992). A pest of Larix in Heilongjiang (Zhao & Zhang, 1992).
Recorded in Korea on Pinus densiflora, Pinus thunbergii, Picea jezoensis and Larix olgensis var. koreana (Park et al., 1999).
Unknown.
China: Heilongjiang (Dailing Mts; Muling; Lesser Khingan Mts, Fengling Forest); Liaoning (Linjiang); Shaanxi (Taibai Shan, Heihe National Forest Park, Houzhenzi, 1500m (33°53'N 107°49'E)).
The population in the Taibai Shan of southern Shaanxi Province, China, is isolated from the main population farther to the northeast.
Mongolia Dundgovi Province (Khuld Soum, Dov Dev); Selenge Province (Tunkhel village, Khailaast); Töv Province (Ulaan-Baatar City).
North Korea: North Hamgyong Province (Baekdu-san; Gyungsung; Chongjin).South Korea: Seoul (Nam-san; Cheongyang-ri); Kyonggi Province (Suwon; Suri-san; Gwangleung; Cheonma-san; Chukryong-san; Baekun-san; Asan Bay; Cheonggye-san; Soyo-san); Kangwon Province (Seolak-san; Odae-san; Chiak-san; Baekduk-san; Taebek-san; Donghae; Yangyang; Chupungryung; Wolak-san; Songni-san; Minjuji-san; Jeonju; Daedun-san; Jiri-san; Namwon); South Cholla Province (Baekyang-san; Gwangyang); South Kyongsang Province (Baekun-san; Yeohang-san; Jinju; Pusan; Geoje-do; Goseong; Sacheon; Sancheong; Yangsan; Jinyang; Hadong; Hamyang; Hapcheon).
Japan: Hahajima (Konokiyama); Tsushima (Inoue, 1977).
Russia: Altai (Cherga; Yailyu; Barnaul; Souzga); Siberia (Krasnoyarsk; Tomsk; Kalinovka; Korolevka; Bazoy; Chingisy; Novosibirsk; Kalinovka); Buryatia (Temnik; Shumilikha (Barguzin Nature Reserve); Baikalskii Nature Reserve; Nizhneangarsk; Verzhnyaya Zaimka; Muya; Verkhnyaya Zaimka; Mondy; Zun-Murino; Vydrino; Taezhnyi; Dabatui; Murochi; Dabatui); Transbaikalia (Lamsky Gorodok; Chita; 20 km N Chita; Mogocha; Tupik; 60 km SE Amazar; Sokhondinskii Nature Reserve (Agutsa, Nizhnii Bukukun); Ur'upino); Amurskaya (Teply; Belogorsk; Blagoveshchensk; Zeiskii nature reserve; Tynda; Ekimchau; Koboldo; Kuravinskoye); Khabarovskiy Krai (Bolshekhekhtsyrskii Nature Reserve, Khabarovsk suburbs; Nikolayevsk-na-Amure; Kiselevka; Botchinskii Nature Reserve; Tumninsky Nature Reserve; northern Bureinskii/Bureya Hills); Primorskiy Krai (Khasan; Vladivostok; Sikhote-Alin, Kamenushka; Teplyi Klyuch; Ussuriysk; Livadia, near Anisimovka); Sakhalin Island.
This subspecies occurs from central Russia (Krasnoturyinsk, southern Urals (Ilya Svetlov, iNaturalist 2024), the Ob' river valley and Krasnoyarsk) (Danner et al., 1998), and the Altai (Plotnikov & Gninenko, 1980; Svyatoslav Knyazev, iNaturalist 2022; Knyazev, 2023), eastward through Mongolia (Puntsagdulam et al., 2005; Enkhtur, Brehm, Boldgiv & Pfeiffer, 2021) and northeastern China, to Primorskiy Krai, Sakhalin Island, the Korean Peninsula and Tsushima, Japan.
In eastern european Russia the distribution of this species now overlaps with that of Hyloicus pinastri in the southern Urals, especially around Chelyabinsk and Yekaterinburg.
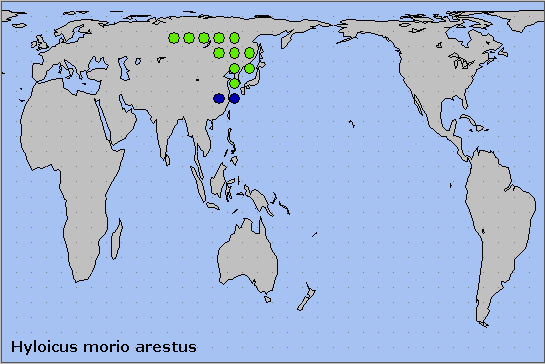
Holarctic; eastern Palaearctic region. Pleistocene refuge: Monocentric -- Manchurian refugium.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list