
Triptogon oriens Butler, 1875, Proceedings of the Zoological Society of London, 1875: 255. Type locality: NE India.
Synonym. Triptogon fuscescens Butler, 1875.
Synonym. Triptogon javanica Butler, 1875.
Synonym. Triptogon massuriensis Butler, 1875.
Synonym. Triptogon oriens Butler, 1875.
Synonym. Triptogon silhetensis Butler, 1875.
Synonym. Triptogon sinensis Butler, 1875.
Synonym. Triptogon andamana Moore, 1877.
Synonym. Marumba dyras plana Clark, 1923.
Synonym. Marumba dyras handeliioides Mell, 1937.
Synonym. Marumba dyras sumatrana Gehlen, 1940.
Synonym. Marumba dyras tonkinensis Clark, 1936.
Note. In an analysis of Marumba dyras, Haxaire & Melichar, 2023, split the previously accepted population of Marumba dyras dyras into two subspecies. In the male genitalia, examples from Sri Lanka and southern India have a narrow, spatula-shaped gnathos of approximately the same width from base to apex. In contrast, specimens from northern India (and beyond) have a gnathos that is at least twice as wide at the base, then sharply narrowed about halfway along to form a bluntly triangular apex. The uncus of southern insects is also more triangular, with slightly tapered sides. DNA barcodes confirmed this split. Indian Marumba dyras comprised two distinct BINs, BOLD:AAF0756 in the south, and BOLD:AAU0213 in the north, with a genetic divergence between them of 4.2% on average. This value is consistent with subspecific differentiation in Smerinthinae (Haxaire & Melichar, 2023). Those in Sri Lanka and southern India remained as Marumba dyras dyras, those farther north were reassigned to Marumba dyras oriens.
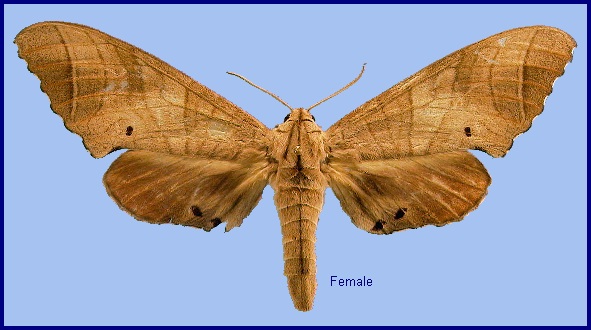
Wingspan: 90--125mm. Forewings vary from earthen-brown to pale grey with brown markings. In the the most distal double line(s) the external one is much heavier than the proximal one; the former stops mostly at Cu1, but occasionally continues beyond, curving basad and ending at the marginal spot; the inner line encircles the spot Cu2, its posterior portion often very faint; there is sometimes a chocolate-coloured area along outer margin. Hindwing reddish to yellowish, with fuscous base and large anal spot. Tongue with fringe. Pilifer with brush of bristles. Antenna one-third length of forewing in male, a little shorter in female (Bell & Scott, 1937).
In the male genitalia, uncus divided into two rounded lobes. Gnathos with pointed medial lobe varying in shape. Valva with dorso-apical lobe pointed, shorter than in Marumba sperchius; subdorsal basal fold with long process. Harpe more strongly hook-shaped, intermediate between Marumba sperchius and Marumba amboinicus, not denticulate. Phallus with processes long, granulose. In the female genitalia, sterigma with prominent rounded lobe in front of ostium bursae.




Prefers thickly wooded areas with heavy rainfall. The moths are rarely attracted by light, and do not feed. They do not mate readily in captivity and wild males do not come readily to bred females (Bell & Scott, 1937).
China: ii-xi (Hong Kong); iv (Guizhou); 22.iv-4.viii (Hunan); vi (Guangdong); vii (Shaanxi; Guizhou; Xizang/Tibet); viii (Fujian; Yunnan); ix (Shaanxi).
Kendrick (2002) states that it is multivoltine in Hong Kong, occurring from February until November, with peaks in early April, late June, late July-mid August, and early October.
OVUM: Very pale green or yellow, slightly depressed ovoid (2.5 x 2.0 x 1.5mm), surface smooth to the naked eye, but under a strong lens irregularly superficially pitted (Bell & Scott, 1937).
LARVA: Full-fed 80mm, width 13mm, horn 14 mm. According to Bell & Scott (1937), in the first instar head round and large; body long and thin, cylindrical; horn long, straight, tip shortly bifid; the ends of the anal claspers extending behind the point of anal flap. Surface of head dull, covered with minute tubercles; body the same dull colour as the head and body yellowish-white. The basal three-fifths and the terminal fifth of the horn black, the middle fifth pale yellow; spiracles white. In the second instar, head triangular with a short process rising from the vertex of each lobe, the two processes closely appressed except for a short distance near the tips. Surface of head shiny and smooth except for two lines of prominent pointed tubercles on each side, one line running from near the vertex of each lobe down the side of the clypeus and the other separating the face from the cheek; the latter with a few scattered tubercles. Body long and cylindrical; horn straight, of medium length, tip shortly bifid. A line of small conical tubercles encircles each secondary ring of the body. There is also a line of larger tubercles on the oblique lateral stripes, some of these with two or three points. Colour of head and of dorsum of body yellowish-green, venter of body neutral tint; horn maroon, the base pale green, a ring of pale yellow two-thirds from base. The third instar is very similar to the second instar, as is the fourth.
In the fifth instar, head large, triangular, with a very short process on the apex of each lobe; face slightly convex; true clypeus one-third length of head, which forms an equilateral triangle with the basal angles rounded and tumid. Apex of false clypeus forming a narrow arch over the apex of true clypeus; labrum half as long as slightly broader than clypeus, narrowing frontad, with six longitudinal ribs, hind margin arched strongly backwards. Ligula kidney-shaped, as long as and half as broad as labrum. Surface of head dull, covered sparsely with small conical, shiny tubercles, those on the cheek slightly larger than the rest. Body shaped as in others of the genus. Horn of medium length, straight, tapering evenly to a blunt point. Surface of body dull, with line of small, conical tubercles encircling each secondary ring. There is a subdorsal line of larger tubercles on segments 3 and 4, and a line of large tubercles along each oblique lateral stripe. Horn covered with small tubercles (Bell & Scott, 1937).
In colour, head pale bluish-green; basal angles of clypeus orange; labrum glassy-green; ligula opaque whitish; antenna very pale green, the end-segment tinted with rose; mandible pale peach-colour, tip dark reddish-brown. Body varying from bluish-green to yellowish-green or greyish-green, with some individuals even being yellow. In the greenish forms the subdorsal and oblique lateral stripes are narrow and yellow; the tubercles bluish. The horn is yellow, and sometimes yellow patches occur below the oblique stripes. In the yellow form the stripes and tubercles are of a maroon-rose colour, the horn orange or reddish; the true legs pink, or orange-banded and blotched with red. Sometimes the orange/red pigmentation in this yellow form is very extensive and may extend out over most of the body. Spiracles oval, lilac, rose-colour or reddish-brown, the central slit pure white, the whole with a narrow pale green rim (Bell & Scott, 1937).
When large the larva usually eats straight across the leaves.






PUPA: 47--52mm, width 16mm. Colour dark red-brown, the frontal ridges and the tumid anterior portions of the segments darker; spiracles and cremaster black. Similar in shape to others of the genus. Frons with six transverse, very rugose, shiny ridges on each side of the dorsal line and close to it, the anterior ridge the highest, forming horn-like projections from the front of the head. Dorsal line of the head and segment 2 rising at a steep angle to the longitudinal axis of the body, the front part of 2 tumid and its dorsal line slightly carinate. Thorax short; tongue broad and short, ending before middle of wing-case. Antenna shorter than fore leg, which reaches to middle of wing-case; mid-leg reaching to about two-thirds the length of wing-case. Surface of head, thorax and wing-case very superficially corrugate-aciculate; abdomen obscurely corrugate, the front quarter of each of segments 5 to 12 very deeply, coarsely, longitudinally pitted and corrugate. There is one ante-spiracular ridge on the front bevel of 9, with a channel behind it. Segment 10 with a similar but smaller ridge and channel, and 11 with the ridge and channel nearly obsolete. Spiracle of 2 indicated by a slit at the junction of 2 and 3, the hind margin of 2 being raised in front of it and a narrow up-tilted lobe projecting from the front margin of 3 bordering the slit behind, the other spiracles parallel-sided, the ends broadly rounded, the whole slightly raised, with the central slit depressed. Cremaster triangular in shape, broad at base and ending in a short, oblong, minutely bifid tip; the upper surface very coarsely longitudinally and transversely corrugate and pitted, the whole surface shiny. The clasper scars on segment 14 prominent; in the male pupa the sex-mark on 13 nearly as long as that segment; round, with thickened lips and a depressed central slit; in the female pupa the sex-marks consist of a depression in the middle of 12 and a pit in a forward prolongation of 14 which runs across 13 to near the middle of 12 (Bell & Scott, 1937).
The pupa when touched, moves the tip of the abdomen round and round, and also makes a 'shivering' motion by rapid contractions and expansions of the abdomen.

Larval hostplants. Recorded in Guangdong/Hong Kong on Byttneria aspera, Firmiana simplex [syn. Sterculia platanifolia], Microcos paniculata [syn. Grewia micrococos], Pterospermum heterophyllum and Sterculia lanceolata (Mell, 1922b; Li, pers. comm. 2002); Chu & Wang (1980) also listed Tilia tuan. For Hong Kong, Tennent (1992) gave Hibiscus mutabilis and Microcos paniculata. Generally oligophagous on trees of Bombacaceae, Malvaceae, Sterculiaceae and Tiliaceae (all of which have recently been combined into a more inclusive family Malvaceae within the order Malvales; Judd & Manchester, 1997). However, in India, M. dyras dyras has been recorded on Bridelia (Euphorbiaceae; Bell & Scott, 1937), Sapindus and Schleichera (both Sapindaceae; Beeson, 1941, and Bell & Scott, 1937, respectively). The record on Cajanus (Fabaceae; Pholboon, 1965) is highly suspect.
In Taiwan, recorded from Firmiana simplex, Pterospermum acerifolium and Sterculia nobilis.
In Laos and Thailand, recorded from Bombax anceps, Hibiscus rosa-sinensis and Microcos paniculata (Eitschberger & Ihle, 2008).
Unknown.
China: ?Liaoning; ?Hebei; ?Shaanxi (Xian; Xunyang, 1380m); Jiangsu; Anhui (Mt. Huang Shan; Anqing; Zhanling); Zhejiang (Tianmu Shan; Kuocang Mountain Nature Reserve); Sichuan (Pengshui; Xinshan); Chongqing (Simian Mountain National Scenic Resort); Yunnan (Changning Co., Songzhishanding, 2800m; Nabanhe National Nature Reserve, Xishuangbanna); Xizang/Tibet (Xiachayu, Zayu County); Guizhou (Taojiang; Jiucai Ling; Xinzhaidashan, Zhijin County, 1000-2000m); Hunan (Hengshan; Daweishan); Jiangxi; Fujian (Longqi Shan; Guangze, 1200m); Guangdong (Nanling National Forest Park, 1100m (Morishita & Kishida, 2000)); Macau; Hong Kong (Shek Kong; Quarry Bay); Guangxi (Maoer Shan, 1800m); Hainan (Sanya, Mt. Jianfengling; Duowen Ling, nr Lingao; Longhushan, Wenchang City).
Taiwan: Taichung Hsien (Tungpu) (Inoue, 1990).
Widespread throughout tropical and warm sub-tropical continental South East Asia, from northwestern India (Chandra, Pandey, Bhandari & Sambath, 2013; Pathania, Sunita Sharma & Gill, 2014; Chakraborty, Chakraborty, Biswas, Chakraborty & Deb, 2024), east through Nepal, Bhutan (Irungbam & Irungbam, 2019), Burma/Myanmar, Andaman and Nicobar Islands (Kailash Chandra & Rajan, 2004; Singh, Ahmad & Chandra, 2021), southern China (north as far as ?Shaanxi and Jiangsu), Thailand, Vietnam (Le & Vu, 2024), Peninsular Malaysia and Taiwan. Wang (1993) and Wang & Zhao (1993) listed Marumba dyras oriens from Liaoning and Hebei but these northern records should be considered unconfirmed until specimens are examined.
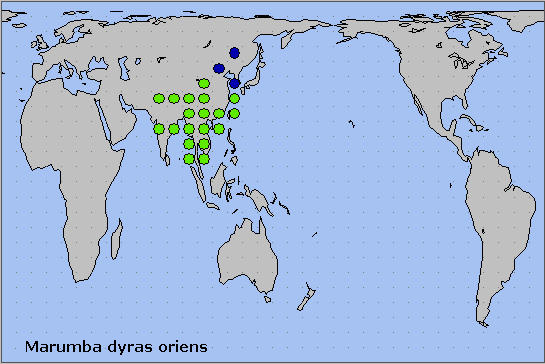
Holarctic and Oriental; eastern Palaearctic and Oriental regions. Pleistocene refuge: Unknown.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list