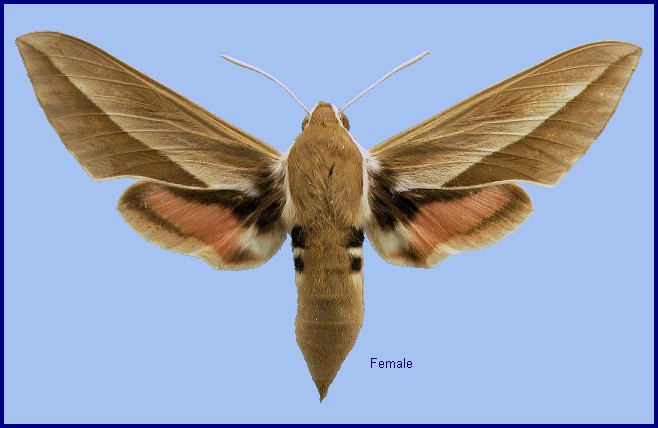
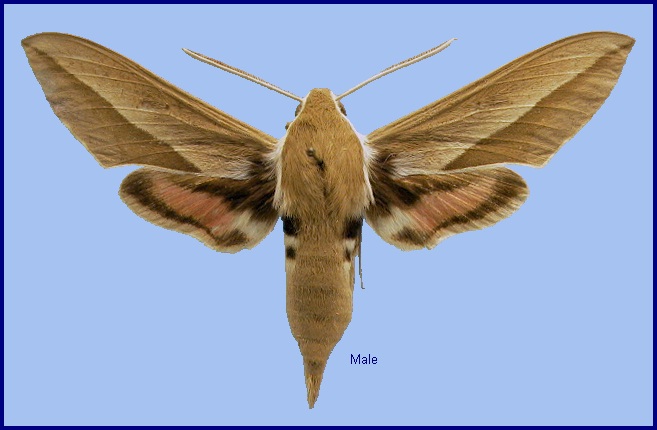
Deilephila bienerti Staudinger, 1874, Stettin. ent. Ztg 35: 91. Type locality: Northeast Persia [Iran], Schahrud [Emamrud].
Synonym. Deilephila bienerti Staudinger, 1874.
Synonym. Deilephila insidiosa Erschoff, 1874.
Synonym. Hyles hippophaes caucasica Denso, 1913.
Synonym. Celerio hippophaes caucasica Clark, 1922.
Synonym. Celerio hippophaes ornatus Gehlen, 1930.
Synonym. Hyles hippophaes transcaucasica Gehlen, 1932.
Synonym. Celerio hippophaes anatolica Rebel, 1933.
Synonym. Celerio hippophaes bucharana Sheljuzhko, 1933.
Synonym. Celerio hippophaes shugnana Sheljuzhko, 1933.
Synonym. Celerio hippophaes malatiatus Gehlen, 1934.
Synonym. Celerio hippophaes baltistana O. Bang-Haas, 1939.
Synonym. Hyles hippophaes miatleuskii Eitschberger & Saldaitis, 2000, Atalanta 31: 213.
Note. This subspecies can be very variable in both coloration and size where numerous climatic conditions occur in close proximity to each other, such as in mountainous areas. Many of these forms were described as distinct subspecies but this is not warranted. Subspecies ornatus, transcaucasica, anatolica, bucharana, shugnana, malatiatus, caucasica and baltistana have been synonymized with subsp. bienerti and should be regarded as forms, although there may be some justification in retaining baltistana (semi-isolated in NE Afghanistan, Pakistan and NW India) as a good subspecies. However, Patzold, Marabuto, Daneck, O'Neill, Kitching & Hundsdoerfer (2021) found no evidence for a separate mitochondrial lineage in baltistana, nor consistent phenotypic differences, to separate this taxon from Hyles hippophaes bienerti.
Note. With regard to Hyles chamyla, there is mounting evidence to suggest that Hyles chamyla f. apocyni may be a hybrid between Hyles chamyla and Hyles hippophaes bienerti (Patzold, Marabuto, Daneck, O'Neill, Kitching & Hundsdoerfer, 2021). The two taxa appear to form a population group that is in the process of speciation, with ongoing admixture competing against continuous ecological differentiation. However, they did conclude that further research is needed, including of nuclear data, to clarify the origin, development and maintenance of the pattern they found.
Wingspan: 65--80mm. Considerably paler and browner than related subspecies. A pale, oblique median line is noticeable on the underside of the forewing; hindwing patches more orange than red. Some large specimens found above 2000m in north-west Iran and Kashmir tend to f. caucasica in coloration.
Often common in mountainous, arid steppe, especially along rivers overgrown with Hippophae or Elaeagnus. Although found at any altitude from 400--3000m, most populations occur from 1000--2000m where Hippophae rhamnoides often forms discrete thickets away from rivers.
China: 29.iv-5.v (Shihezi); 31.v-13.vi (Shihezi); 20.vi (Shaanxi); 1.vii (Zada); vii (Yining/Gulja, 1000m); 5-31.vii (Shihezi); 28.ix (Dingbian). Mongolia: 3-19.vi (Khovd Province); 19.vii (Khovd Province). Russia: 19-20.vi (Karasuk, Novosibirsk area); 14.vii (Tuva); 18-21.vii (Altai); 30.viii (Karasuk, Novosibirsk area).
There are two generations a year in northeastern and central China, with adults flying in May and July/August (Yang, 1978; Chu & Wang, 1982). In Xinjiang, the first brood emerges between late April and mid June, depending upon the weather.
OVUM: Pale greenish-grey, almost spherical (1.0 x 1.1mm). Deposited on both the upper and under surface of leaves, usually near the edge, on the lower branches of the hostplant. Thicket-edge or isolated shrubs are preferred, with up to 500 being laid by each female.
LARVA: Full-fed 75--80mm. Dichromatic: unstriped or striped.
On hatching, the eggshell is ignored, the 3--4mm-long larva proceeding to find a resting place below a leaf, a site to which it returns after each spell of feeding. At this stage it is dark green, thickly speckled with white and dark grey. The final instar has two colour forms. One is dark green (in some cases suffused with pink), thickly speckled with white and grey; superimposed on this are an off-white dorso-lateral line, often with orange eye-spots, and a broader, white, ventro-lateral stripe running just above the legs. Horn long, thin, orange below, black above, with two elongated orange spots at its base; head green, with two brown lines. The other colour form is silvery grey, with a black, broken dorso-lateral line from which emanate black, equally broken oblique lateral stripes with white, red, or yellow patches often present in between. Head brown and grey; horn as above. In a very rare colour form, all green coloration is replaced by pinkish brown.
Larvae frequently sun themselves openly on the upper branches, amongst those they have already stripped of leaves. There is a very heavy mortality due to parasitoids. Those that survive eventually become light brown before descending to find a pupation site, often after hours of perambulation on the ground.
This stage lasts as little as 28 days, during which the larva basks quite openly on the topmost branches of its hostplant.

PUPA: 40--50mm. Pale yellowish brown, or light grey-brown, with dark brown striations. More elongated than others of the genus. Enclosed in a flimsy cocoon amongst roots or under stones. The overwintering stage.
In the summer months it remains in this stage for no more than 20 days. Formed in a chamber in the soil, often up to 10cm deep (Chu & Wang, 1980b).


Larval hostplants. Recorded in China on Elaeagnus angustifolia (sensu lato) and Hippophae rhamnoides (Wang, 1978; Pittaway & Kitching, 2000), which are also the main hostplants in Tajikistan (Shchetkin, 1956).
Unknown for China.
China: Xinjiang (Shihezi; Ürümqi; Yining/Gulja; Manas County); Nei Mongol (Alxa Zuoqi; Alxa Youqi; Ordos City); Liaoning; Shaanxi (Dingbian; Yulin); Ningxia (Yinchuan; Lingwu; Pingluo); Gansu; Xizang/Tibet (Zada, 2950m).
Mongolia Khovd Province (Bodonchijn River valley, Elkhony-Ekhen-Tal, 30km S Altai village, 1200m (45°43'N 92°05'E); Dzun-Dzhargalant-Khairkhan, Ar-Shatyn-Gol (River) Valley, 2100m (47°44'N 92°27'E); Bulgan River basin, Bayan River valley, Ulyastayn-Sala River, 1900m (46°21'N 91°08'E); Bulgan River valley, 45km N Bulgan, 1500m; Dzhungarian Gobi, 45km SW Bulgan, Uvhod-Ula Mts., 1350m); Ömnögovi Province (Dalanzadgad, Shatiin am).
Russia: Altai (Cherga; 5km SWW Mikhailovskoe); Western Siberia (Karasuk, Novosibirsk area); Tuva (Khovu-Aksy); Transbaikalia.
Moldova (Derzhavets, 1984; Tugulea, 2016; Khalaim, 2022), Romania (Esper, [1793]; Székely & Szabó, 1995; Vlad Dinca, pers. comm. 2007; Manci, Sitar, Corduneanu & Balan, 2015; Khalaim, 2022), Bulgaria (Beshkov, 1998; Danner, Eitschberger & Surholt, 1998), northern Greece (Koutsaftikis, 1970; 1973; 1974), the Aegean Islands (de Freina & Piatkowski, 1999), western Turkey (Pittaway, 1982a), central (Rebel, 1933), south-eastern and eastern Turkey (Daniel, 1932; Daniel, 1939; de Freina, 2012; Kemal & Koçak, 2014; Kemal & Koçak, 2016; Koçak & Kemal, 2018), southern Ukraine (Efetov & Budashkin, 1990; Zolotuhin, pers. comm.; Vasilyuk & Inozemtseva, 2003; Khalaim, 2022), the Caucasus and southern European Russia (Zolotuhin, pers. comm; Poltavsky, pers. comm. 2003; Anikin, 2004), the Republic of Georgia (Didmanidze, Petrov & Zolotuhin, 2013), Armenia (Wąsala & Zamorski, 2015) and Azerbaijan (Didmanidze, Petrov & Zolotuhin, 2013; Snegovaya & Petrov, 2021), northern and central Iran (Bienert, 1870; Barou, 1967; Kalali, 1976; Ghassemi, Alemansoor & Alehossein, 2009; Lehmann & Zahiri, 2011), Turkmenistan (Danov & Pereladov, 1985) and Uzbekistan (Derzhavets, 1984), the southern Urals to Omsk (Nupponen & Fibiger, 2002; Dubatolov, 2012; Knyazev, 2020), eastern Kazakhstan (Kondratiev coll., NHMUK; Shovkoon, 2015), western Xinjiang Province, China (Pittaway & Kitching, 2000), Tajikistan (Shchetkin, 1956), Afghanistan (Ebert, 1969; Daniel, 1971), northern Pakistan - Gilgit–Baltistan (Khalti Lake, 10.vi.2008), northern Pakistan - Azad Kashmir (Karakoram Mountains, Juglot Valley, 2550m, 26.vii.2011 (leg. Balázs Benedek) (Rafi et al., 2014)), and north-west India - Jammu & Kashmir (O. Bang-Haas, 1939; Sidhu, Nair & Kubendran, 2018) and Himachal Pradesh (Sidhu, Nair & Kubendran, 2018), to the western Tian Shan.
Also, China, from Xinjiang Province (China) north to the Altai Mountains (Izerskiy, 1999) and eastwards across the provinces of Ningxia, Gansu, Shaanxi and Nei Mongol (Inner Mongolia) to Liaoning (Chu & Wang, 1980b; Pittaway & Kitching, 2000), and also Mongolia (Derzhavets, 1977; Yakovlev, Gus'kova, Doroshkin & Titov, 2015; Yakovlev & Doroshkin, 2017; Enkhtur, Brehm, Boldgiv & Pfeiffer, 2021). From these areas north to Karasuk (Dubatolov, 2012), the Tuva A.S.S.R. (Viidalepp, 1979; Izerskiy, 1999), the Altai Krai (Yakovlev, Dubatolov & Titov, 2013) and Lake Baikal (Kondratiev coll., NHMUK) in Russia.
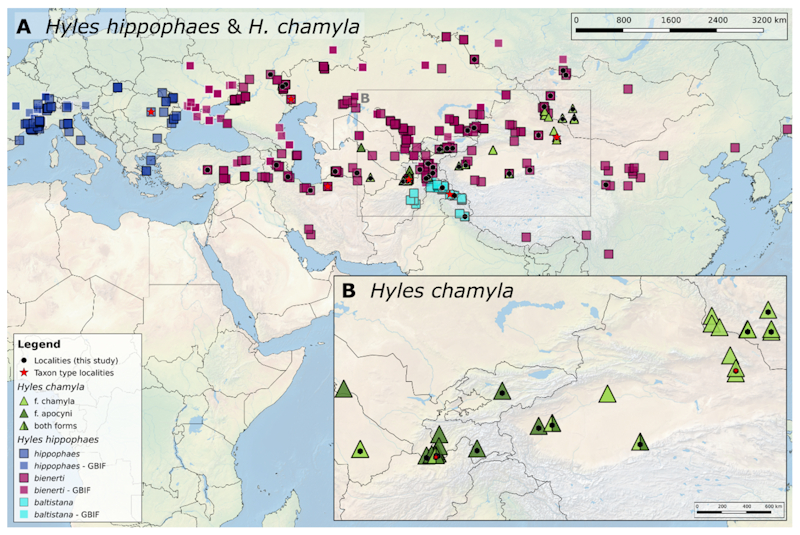
Map: Distribution of Hyles hippophaes and Hyles chamyla in Europe and Asia. Blue of differing shades and magenta squares mark occurrence points of the three infraspecific taxa associated with H. hippophaes; green triangles mark occurrence points for the two H. chamyla forms; black dots indicate genetic samples used in this study; red stars mark the type localities of the taxa; white borders indicate data points derived from GBIF. (© Eduardo Marabuto, in Patzold, Marabuto, Daneck, O'Neill, Kitching & Hundsdoerfer, 2021).
Holarctic; Palaearctic (both eastern and western subregions). Pleistocene refuge: Polycentric -- Caspian, Iranian, Turanoeremic, Turkestan and Mongoloeremic refugia.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list