


Macroglossa affinis Bremer, 1861, Bull. Acad. imp. Sci. St Pétersb. 3: 475. Type locality: [Russia, Khabarovskiy Krai] Ussuri, <<between the mouth of the Ussuri river and the Noor [He] river>>.
Synonym. Macroglossa affinis Bremer, 1861.
Synonym. Macroglossa sieboldi Boisduval, 1869.
Synonym. Sesia alternata Butler, 1874.
Synonym. Sesia whitelyi Butler, 1874.
Synonym. Macroglossa ganssuensis Grum-Grshimailo, 1891.
Synonym. Macroglossa confinis Staudinger, 1892.
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan.]
Wingspan: 43-54mm. Very similar to Hemaris fuciformis but distinguishable by the darker wing borders, thinner scaling on the apical crossveins of the forewing cell and the conspicuous pale margin to the thorax. As with all other members of this genus, the adult emerges from the pupa with wings fully scaled. The hyaline areas only appear after the first flight after the scales are shed.
In the male genitalia, phallus processes longer than in Hemaris fuciformis and not quite so broad.



Diurnal. A woodland species, but preferring the margins and clearings along rivulets, where it flies all day (Izerskiy, 1999b; Dubatolov, pers. comm. 2010); here, they often drink from the waters' surface during flight (Dubatolov, pers. comm. 2010). However, this species has adapted well to the bigger parks and gardens of Beijing, where it can be found breeding in the shrubberies under trees (Pittaway, pers. obs. 2003).
In the Russian Far East, in late May and early June, adults are active mostly from 08:00 until 12:00 a.m. and then again from 14:00 until 18:00 p.m. They prefer to visit for nectar flowers of Syringa, Lonicera, Rhododendron dauricum, Deutzia amurensis, Filipendula and Stellaria. Pairing usually takes place on the first day after enclosing from the pupae, and lasts about 30-40 minutes. This can even take place in flight, especially while feeding from flowers. Copulating pairs can fly, with females continuing to feed (Koshkin & Yevdoshenko, 2019).

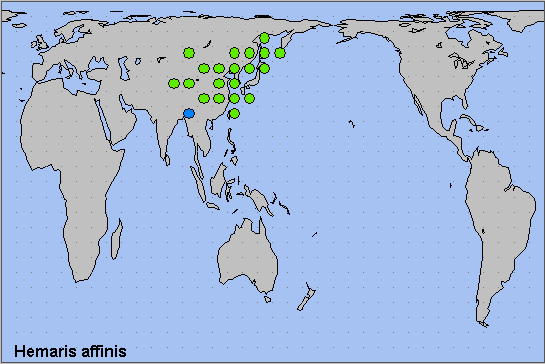
China: iv (Beijing; Shandong); v (Gansu); vi (Jiangsu; Sichuan; Qinghai); vii (Chongqing; Gansu; Hubei; Heilongjiang); vii-viii (Shandong); viii (Zhejiang); 2.ix (Beijing). Taiwan: v ([unstated locality]); vi (Nantou Hsien). Japan: iv-viii (Honshu); v (Shikoku); 4.vi-23.viii (Hokkaido). Russia: 25.v-26.vii (Khabarovskiy Krai); 5.vi-15.vii (Primorskiy Krai); 3.viii (Primorskiy Krai).
In northern China there are two generations per year, with adults flying between May and late August (Yang, 1978).
Park et al. (1999) give early May until early November as the flight period in Korea.
Bivoltine in the Russian Far East, where it is common. Flight period from mid May until late June (first generation) and then again from early July until late August (second generation). The latter can partly overlap with the first in time (Koshkin & Yevdoshenko, 2019).
OVUM:
LARVA: In the fifth and final instar, the ground colour of the body and head is bright or light green, with a large number of small rounded yellowish or whitish granules/spots; there are no bodily setae. Two indistinct whitish lines run down the entire dorsal surface, one either side of the green dorsal aorta. A yellowish or whitish dorso-lateral line is present, beginning at the anterior edge of the mesothorax and running uninterrupted to the base of the caudal horn. Sometimes, this line is very faint. Spiracles are orange in the center, dark brown on the edges and with a small white dot at each dorsal and ventral end. The ventral surface of the body is dark brown. True legs are light reddish-brown; prolegs are dark brown. The caudal horn is straight or slightly curved, light bluish, or pink, with a large number of black conical granules (Koshkin & Yevdoshenko, 2019). There is also a dark form, with all green coloration replaced by brown.
Found under the terminal twigs of mid-level branches 0.5-1.5m off the ground, never those on top of a shrub. Common in and around Beijing on ornamental shrubs of Lonicera maackii during late August/September and into October (Pittaway, pers. obs. 2003 & 2013).




PUPA: 33-35mm. Very dark brown, almost black, but mahogany brown between the abdominal segments; tapering caudad from a forward projecting head. Head not tuberculate, but with two small downward-pointing sharp hooks frontad. Proboscis not produced and lying flush with and between the slightly carinate wing-cases. Wings and abdominal segments very finely punctate so as to tone down the gloss. Cremaster broadly triangular, dorso-ventrally flatted, with a sharp point; tuberculate. Formed in a loose brown cocoon among debris on the soil. The overwintering stage.


Larval hostplants. Chistyakov & Belyaev (1984) and Nikolay Ivshin (pers. comm. 2020) give Lonicera maackii for the Russian Far East; Koshkin & Yevdoshenko (2019) also list Lonicera caerulea and Lonicera ruprechtiana from the same region. Recorded in Korea on Lonicera japonica and Patrinia scabiosaefolia (formally Valerinaceae, now Caprifoliaceae) (Park et al., 1999), while in Japan it has also been found on Weigela (Chistyakov & Belyaev, 1984). In the gardens of Beijing Lonicera maackii is the main host (Pittaway, pers. obs. 2003).
Will investigate and lay eggs on Symphoricarpos in gardens (Pittaway, pers. obs. 2005).
China: Heilongjiang; Liaoning (Shenyang); Bejing (Haidian; Xiangshan; Botanical Garden; Baihua Shan); Tianjin; Shandong (Yantai; Qingdao); Qinghai (Xining; Haidong); Gansu (nr. Min Xian, 3200m; Maijishan, 1500m); Jiangsu (Nanking); Anhui (Mt. Huang Shan; Hongchong, Lu'an); Zhejiang (Tianmu Shan; Mogan Shan); Hubei (Changyang); Sichuan (Zhongjiang); Chongqing (Jinfo Shan); south Xizang/Tibet (Bomi, 2750m); Fujian (Liufang); Hong Kong.
Taiwan: Nantou Hsien (Jinyüeh Tan).
Mongolia: Changai Mts.
North Korea: Kangwon Province (Daeseong-san); South Hamgyong Province (Wonsan); North Hamgyong Province (Musan).
South Korea: Seoul; Bukhan-san); Kyonggi Province (Dobong-san; Suri-san; Gwangleung; Imjingak; Chukryong-san; Aeng-mubong; Cheonma-san; Paldang; Soyo-san; Mugab-san; Myungji-san); Kangwon Province (Seolak-san; Odae-san; Yangyang; Balwang-san; Chiak-san; Chuncheon; Hoenggye; Oksu Temple; Baeduk-san; Palbong-san; Sangyong); South Chungchong Province (Gyeryong-san); North Cholla Province (Jiri-san; Naejang-san; Namwon); South Cholla Province (Mudeung-san; Wolchul-san; Baekun-san; Jogye-san; Seonam Temple; Jin-do; Wan-do); North Kyongsang Province (Sobaek-san; Dalseong; Youngyang; Geumleung; Mungyang; Andong; Ulleung-do; Uiseong); South Kyongsang Province (Pusan; Geumjung-san; Geoje-do; Namhae; Sancheong; Ulsan; Jinju; Hadong); Cheju Province (Ara-dong; Ora-dong; Mokseokwon; Bijarim; Sunheul; Topyung; Susan-ri; Suakbong; Ipseok-dong; Andeok; Gwaneum Temple; Sungpanak).
Japan: Hokkaido (Hakodate; Kushiro; Tokachi); Honshu (Chichibu; Kami-yoshida; Oiwake; Tokei-ji; Gifu; Nikko; Karuizawa; Hoshikawa; Tokyo; Yokohama; Mt Tsurugi; Mt Asama; Nakauonuma; Mt Mitsumine; Mt Iwamuro); Shikoku (Ino; Kumakogen); Kyushu; Ryukyu Archipelago (Okinawa).
Russia: Southern Amurskaya (Uril; Pashkovo; Svobodnyi; Blagoveshchensk; Arkhara district; Kundur); Yevreyskaya (Obluch'e district; Bastak Nature Reserve; Oktyabrskiy district; Soyuznoe); Khabarovskiy Krai (Bolshekhekhtsyrskii Nature Reserve, Khabarovsk suburbs; Slavyanka; Komsomol'sk-na-Amur; Pivan; Kiselevka; Arkhangelskoe; Botchinskii Nature Reserve; Vyazemsky district; Bikin district; Bureinsky Nature Reserve); Primorskiy Krai (Khasan; Narva; Askold Island; Vladivostok; gora Tsamo-Dynza; gora Chernaya, 1100m; Partizansk district; Tigrovoyi; Anisimovka; Ussuriysk; Vinogradovka; Barabash-Levada; Ryazanovka; Kedrovaya Pad Nature Reserve; Anuchino; near Kalinovka; near Zanadvorovka; Sikhote-Alin Nature Reserve; Pozhiga; Zaretchye village; Kaimanovka; Tayozhka; Lazarevka; Andreevka; Krasnorechensk); ?Sakhalin Island; Kurile Islands (Kunashir Island).
Mongolia, Russian Far East, northern, central and eastern China, Taiwan (Kishida, 1977), North Korea, South Korea, and Japan.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list