

Sphinx elpenor Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 491. Type locality: not stated [Sweden].
Synonym. Sphinx elpenor Linnaeus, 1758.
Synonym. Sphinx porcus Retzius, 1783, Genera Insectorum: 34.
Synonym. Elpenor vitis Oken, 1815, Okens Lehrbuch Naturgesch. 3(1): 760.
Synonym. Chaerocampa lewisii Butler, 1875, Proc. zool. Soc. Lond. 1875: 247.
Synonym. Chaerocampa elpenor cinerescens Newnham, 1900.
Synonym. Eumorpha elpenor clara Tutt, 1904.
Synonym. Eumorpha elpenor obsoleta Tutt, 1904.
Synonym. Eumorpha elpenor pallida Tutt, 1904.
Synonym. Eumorpha elpenor unicolor Tutt, 1904.
Synonym. Eumorpha elpenor virgata Tutt, 1904.
Synonym. Deilephila elpenor vautrini Austaut, 1907.
Synonym. Pergesa elpenor daubi Niepelt, 1908.
Synonym. Pergesa elpenor hades Rebel, 1910.
Synonym. Chaerocampa elpenor alboradiata Lambillion, 1913.
Synonym. Deilephila elpenor philippsi Niepelt, 1921.
Synonym. Pergesa elpenor scheiderbaueri Gschwandner, 1924.
Synonym. Pergesa elpenor lugens Niepelt, 1926.
Synonym. Deilephila elpenor argentea Burrau, 1950.
Synonym. Pergesa elpenor distincta Meyer, 1969.
Synonym. Pergesa elpenor szechuana Chu & Wang, 1980, Acta zootaxon. sin. 5: 421. Type locality: China, Sichuan, Huili.
Synonym. Deilephila elpenor tristis Lempke & Stolk, 1986.
Note. The characters which separate Deilephila elpenor lewisii from Deilephila elpenor elpenor are not confined to that subspecies. Individuals of what are typical subsp. lewisii can be found within the range of subsp. elpenor; the reverse is also true. Subsp. lewisii was therefore synonymized with subsp. elpenor by Pittaway (1993).
Note. Mell (1922) noted that moths from Yunnan, China, were transitional in hindwing pattern between Deilephila elpenor elpenor and Deilephila elpenor macromera, so that the latter may not warrant recognition as a subspecies.
Note. Subsp. szechuana (Chu & Wang, 1980) from Sichuan Province, China, is not valid as it was described from a mouldy specimen, the fungus having dulled the colours (Pittaway & Kitching, 2000). Fresh specimens from Sichuan are indistinguishable from subsp. elpenor.
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan as well as Moths of the southern Shikoku, Japan.]
Wingspan: 52--75mm. A beautiful pink, khaki and black species that cannot be confused with any other species in the region except Deilephila rivularis (Boisduval, 1875). Upperside of forewing khaki, except for costa, a narrow, median band extending from the inner margin to M3, a narrow postmedian band extending from the inner margin to the apex, and the marginal band, all of which are pink. Basal half of hindwing upperside black, distal half pink; distal edge of black area almost straight and parallel to outer margin, so that pink area is of almost even width across the wing. Upperside of head, thorax and abdomen khaki, except for the inner edges and median line of the tegulae, posterior margin of thorax, base of abdomen, abdominal median line and terminal abdominal segments, all of which are pink. Variation is confined to the extent of or, as in the case of f. unicolor Tutt, absence of pink coloration on the forewing.
Male genitalia similar to those of Deilephila porcellus but uncus more slender. Gnathos more rounded distally. Stridulatory scales of valve more numerous. Harpe longer. Apical dentate process of phallus longer.



Hiding away amongst foliage by day, adults do not take flight, unlike many other hawkmoths, until well after dark, when flowers such as Lonicera, Silene, Buddleja and Valeriana are avidly visited. It is a major pollinator of the alpine orchid Habenaria limprichtii in the alpine meadows around Lijiang, Yunnan, China (Tao, et al., 2018). Towards midnight, pairing takes place, with pairs rarely remaining in copula for more than two hours. Following separation, females immediately begin to lay eggs, continuing to do so over a number of nights until approximately 100 have been deposited. It is strongly attracted to light, with large numbers often coming to mercury-vapour lamps between 22.00 and 24.00 hours in England.
In the Russian Far East, a lowland species of mixed and pure deciduous woodland characterized by Quercus mongolica (Izerskiy, 1999b).

China: iv-vi (Jiangsu); v (Shandong; Xinjiang); vi (Xizang/Tibet); 16-25.vi (Zhejiang; Jilin); vii (Heilongjiang; Jilin; Sichuan, 1080m; Yunnan); vii-ix (Liaoning); 21.vii (Hubei); 25.vii (Altai Mountains); viii (Jiangxi; Fujian; Yunnan); 3-31.viii (Zhejiang); ix (Sichuan; Shaanxi); 17-24.ix (Shanghai); 21-28.ix (Zhejiang). Mongolia: 19.vii (Songino). North Korea: vi (Mt. Kuwol; Sinmi-do); vii (Wonsan). South Korea: 28.vii. Japan: 15.v (Shikoku); 11.v-4.vi (Honshu); 27.vii-29.viii (Honshu); 6.vi-30.viii (Hokkaido). Russia: 24.v-14.viii (Khabarovskiy Krai); 25.v-6.vi (Primorskiy Krai); 5.vi-26.vii (Siberia); 22.vi (Sakhalin); 6-7.vii (Altai); 9-16.vii (Khabarovskiy Krai); 17.vi-19.vii (Primorskiy Krai); 18.vii-9.ix (Primorskiy Krai); 31.vii (Altai); 5.viii (Kurile Islands); 12.viii-20.ix (Primorskiy Krai).
Double-brooded in northeastern China, with adults flying between April and October (Yang, 1978). Full-grown larvae are usually met with in July and September (Chu et al., 1979). In neighbouring regions of Russia, Deilephila elpenor elpenor flies in June and July.
Park et al. (1999) give early May until late September as the flight period in Korea.
OVUM: Pale green, almost spherical (1.2 x 1.5mm), shiny and smooth. Laid singly or in pairs underneath the leaves of its hostplant, hatching about ten days later.
LARVA: Full-fed 70--85mm+. Dichromatic: brown or green. [A plate from Butler (1876)]
Newly-hatched larva (4--5mm long) pale green, cylindrical, with a small, narrow horn. In the second instar the head is disproportionately small. With a further moult, the first and second abdominal segments enlarge and have large and extremely realistic eye-spots. It is during this instar that most change to the final dark form, although some remain green. In between feeding, both by day and at night, the young larva rests stretched out beneath a leaf, where it is extremely well camouflaged. Later, larger individuals feed fully exposed at the top of a plant by day or night, preferring flowers and seed-heads to leaves. When not feeding, it often retires to hide at the base of the plant where its dark coloration is of greater advantage. Some larvae, especially those on Galium, may feed openly only at night.
A striking feature of this species is its defensive behaviour. When alarmed, the head and the three thoracic segments are withdrawn into the first and second abdominal segments, which expand greatly, enlarging the startling eye-spots. Even quite large birds have been known to flee at this sight. The coloration of these 'eyes' remains bright until pupation. The larva can also swim if it drops from emergent aquatic hostplants into the water below.



PUPA: 35--47mm. Similar to those of Hippotion celerio and Theretra alecto, but easily distinguished by having a strongly reduced proboscis; brown coloration streaked with even darker brown; and a single row of spines on each mobile, abdominal segment. The function of these has yet to be ascertained, but Brock (1990) has demonstrated that if cocoons of this species are flooded or exposed to high humidity in the UK, up to 90 per cent of pupae rapidly work their way out of the cocoon by using these spines. This behaviour may be an adaptation to avoid drowning during flooding of the habitat. Even so, pupae are very active, often working their way out of their cocoon prior to emergence. Pupation takes place very close to the hostplant in a strongish mesh cocoon amongst debris on the ground. Overwinters as a pupa.



Larval hostplants. Recorded in China on Epilobium, Galium, Impatiens, Lonicera, Lythrum, Pinellia, Rubia and Vitis (Yang, 1978; Chu & Wang, 1980; Lin, 1990; Sheng & Xue, 1993; Pittaway, pers. obs. 2016). Elsewhere, most records are on Balsaminaceae, Lythraceae, Onagraceae, Rubiaceae and Vitaceae.

In Japan, recorded from Impatiens balsamina, Impatiens textori, Lythrum anceps and Vitis.
Recorded in Korea on Oenothera stricta, Impatiens balsamina, Impatiens textori, Epilobium angustifolium, Galium verum var. asiaticum, Colocasia antiquorum and Lythrum anceps (Park et al., 1999).
Ichneumonidae: Amblyjoppa cognatoria (Smith, 1874).
China: Xinjiang (Tian Shan; Yining; Altai Mountains; Yining/Gulja, 900-1200m); Nei Mongol (Ewenkizu; Chen Barag Qi); Heilongjiang (Harbin; Lalin; Zhaodong; Liangshuigou); Jilin (Jiaohe, Lafa Shan; Antu County; Riguangshan Forest Park); Liaoning (Dandong; Huanren; Dalian); Hebei (Guanting Reservoir National Wetland Park; ); Beijing (Liulimiaozhen; Potoucun; Shimenzhen); Shandong (Yantai; Qiangdao; Weihai; Taian); Shaanxi (Xunyang, 1380m); Shanxi; Qinghai (Nangqian County); Henan (Segang); Jiangsu (Nanjing); Anhui (Mt. Huang Shan; Zhechuancun; Hefei); Shanghai; Zhejiang (Tianmu Shan; Shisuntoucun; Wuyanling National Nature Reserve; Yunfengxiang; Longquan; Ruoliao; Daping; Shisuntoucun; Ningbo; Zhangkeng Reservoir; Hangzhou); Hubei (Wuhan; Lichuan; Luotian, 1729m; Tongbaimiao; Wangu Tower; Xiangyang); Sichuan (Emei Shan; Dazhou; Chengdu; Huili; Kangding; Danjingtai; Guantiancun); Yunnan (Changning County, Songzhishanding, 2800m; Gaoligong Shan; Huaping County, Dabaoshan; Lijiang Alpine Botanical Garden, 2725m; Gucheng District, Lijiang); Xizang/Tibet (Jinsha River, Gamtog); Guizhou (Longli County; Qiannan Buyei and Miao Autonomous Prefecture); Hunan (Shangzhi; Lijiawan; Gupoling; Hengshan); Jiangxi (Jiujiang; Nanchang; Hua'ertan); Fujian (Longqi Shan; Guangze, 1200m; Yangjiazhai; Xietun; Wuyishan National Nature Reserve; Ningde; Fuzhou).
Mongolia: Songino.
North Korea: South Hwanghae Province (Mt. Kuwol, 950m); North Pyongan Province (Sinmi-do); South Hamgyong Province (Wonsan); North Hamgyong Province (Ungiryung).
South Korea: Baengnyeong-do & Daecheong-do; Seoul; Kyonggi Province; Kangwon Province; North Chungchong Province; South Chungchong Province; North Cholla Province; South Cholla Province; North Kyongsang Province; South Kyongsang Province; Cheju Province
Japan: Hokkaido (Kushiro; Tokachi); Honshu (Tokyo; Kumanotairo; Mikaboyama, 750m; Iruma; Owa; Kiyosato; Oki Islands (Kadowaki & Kishida, 1977); Asagiri-kogen (Kishida et al., 2018)); Shikoku (Usa); Kyushu; ?Okinawa.
Russia: Altai (Yailyu; Souzga); Siberia (Tomsk; Pozdnyakovo; Tyul'ka; Novodubovo; Krasnoyarsk; Novosibirsk; Chingisy; Alaevo; Kolpashevo; Kolomino; Porotnikovo; Korolevka; Kalinovka; Kharat; Irkutsk); Yakutia/Sakha (Lensk; Olegminsk; Yakutsk; Borogontsy; Khandyga); Buryatia (Baikalskii Nature Reserve; Verkhnyaya Zaimka; Mishikha (Baikalskii Nature Reserve); Baikalskii Priboi; Atsula; Istok; Dzhirga; Maiskii; Monakhovo; Ulan Ude); Transbaikalia (Nizhnii Tsasuchei; 20 km N Chita; Mogocha; Tupik; Argunsk; Shara; Ur'upino; Daurskii Nature Reserve); Amurskaya (Zeiskii nature reserve; Uril; Blagoveshchensk; Zeya; Tynda; Shimanovsk; Norsk, and many other places); Yevreyskaya (Bastak); Khabarovskiy Krai (Khabarovsk; Bolshekhekhtsyrskii Nature Reserve, Khabarovsk suburbs; Slavyanka; Pivan; Kiselevka; Mariinskii Post; Tyr; Arkhangelskoe; Nikolaevsk-na-Amure; Chlya; gora Belaya [= White Mountain]; Botchinskii Nature Reserve; Bureinsky Nature Reserve; Tumninsky Nature Reserve); Primorskiy Krai (Askold Island; Jankowski Peninsula; Lesogor'e; Khasan; Primorskiy; Ussuriysk; Vityaz Bay; Kedrovaya Pad Nature Reserve; near Kalinovka; near Zanadvorovka; near Barabash; Vladivostok; Spassk-Dalny); Sakhalin Island (Urozhaynoje; Novoaleksandrovik); Kurile Islands (Kunashir).
Recorded by Pittaway (1993) as a non-migratory resident that occurs throughout Europe (with the exception of northern Scandinavia, northern Scotland and parts of the Iberian Peninsula), eastward through temperate Russia north of the Kyrgyz Steppe, southern Siberia, southern Yakutia/Sakha (Kaimuk et al., 2005), Amurskaya (Streltzov, Osipov & Malikova, 2003) to the Pacific coast (including Sakhalin and Kunashir Island (Dubatolov, 1991)), Korea and Japan; then south through China as far as Sichuan and Guangdong (Danner et al., 1998) to Bhutan (Dierl, 1975; Irungbam & Irungbam, 2019). In the south, recorded from Turkey, then east through the Caucasus to northern Iran and the Kopet Dagh of Turkmenistan. It is also present in eastern Kazakhstan (Alpheraky, 1882; Danner, Eitschberger & Surholt, 1998), Kyrgyzstan (Korb, 2018) and northern Uzbekistan (Kozhanchikov, 1930) north to the Altai Mountains (Knyazev, 2023).
This species was recorded from north-eastern Afghanistan by Ebert (1969), but in his 1974 publication he points out that this was an error and that the specimen in question was Deilephila rivularis (Boisduval, [1875]).
Deilephila elpenor has also been reported from northern Pakistan (Humairah Hanif et al., 2016). However, the specimens illustrated match the pale form of Deilephila rivularis. Interestingly, Sphinx ligustri was also reported from the same area by the same authors. Both species may have only recently spread south into this area as Bell & Scott (1937) failed to find either, but this requires confirmation. The latter, however, has also been reported from northwestern India (Smetacek, 1994).
Records of this species from Taiwan are erroneous.
This species has also recently been recorded from southern British Columbia (Canada) and northern Washington State (USA), as an introduction.
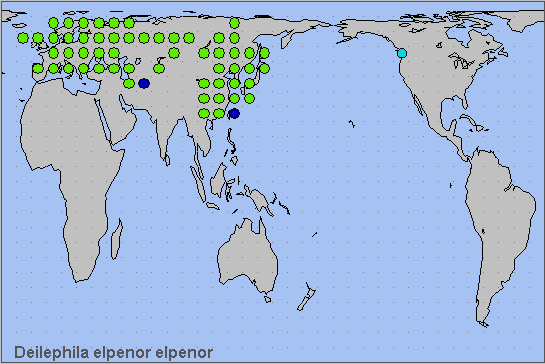
Holarctic; Palaearctic (both eastern and western subregions). Pleistocene refuge: Polycentric -- Holomediterranean, Caspian, Iranian, Turkestan and Manchurian refugia.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list