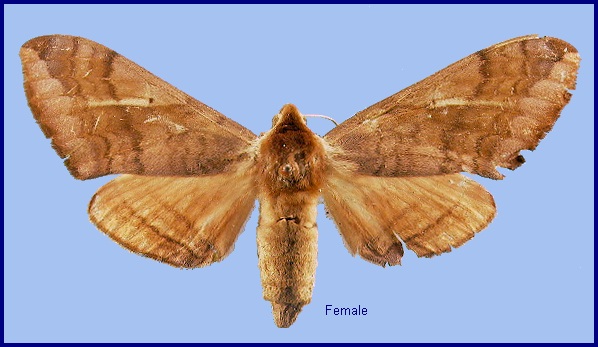
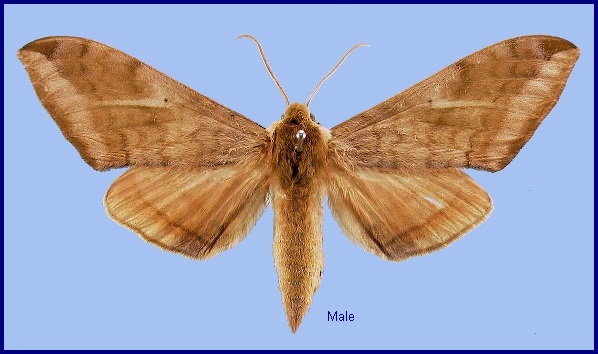
Basiana exusta Butler, 1875, Proc. zool. Soc. Lond. 1875: 252. Type locality: Northwest Himalayas [India, Himachal Pradesh,] Kunawur [Kinnaur].
Synonym. Basiana exusta Butler, 1875.
Wingspan: 70--96mm. Superficially resembling species of Clanis but proboscis much shorter, forewing broader and not falcate apically, tibiae lacking spines, and pulvilli and arolium absent. Uppersides of mid- and hindtibiae greyish-white. Forewing underside lacking a black streak posterior to the discal cell. Hindwing upperside lacking the black basal patch present in many species of Clanis.
The the male genitalia, uncus broad, tapered towards apex, which is sinuate with obtuse lobes. Gnathos with a broad triangular medial lobe that is somewhat constricted basally. Valve sole-shaped, lacking stridulatory scales and the basal tuberculate lobe found in Clanis. Harpe represented by a weak, longitudinal medial fold, ending in a flat, spatulate, downcurved process, and lacking. Phallus weak, lacking armature.


China: 4.vii (Xizang/Tibet).
In Nepal from mid May to June at 1550-1700m altitude. The single individual from Bhutan was found on 11.vi.2018 at 906m altitude (Jamtsho & Irungbam, 2019).
OVUM: Green, oval, dull but smooth. Very large for the size of moth (Bell & Scott, 1937).
The large eggs are sometimes laid singly, but more often in pairs, on the leaflets of Indigofera (Fabaceae). As the leaves of this plant close in the evening, the eggs must either be laid early or the leaflets forced apart by the moth while ovipositing (Bell & Scott, 1937).
LARVA: Full-fed 95mm. In the first instar, head round and of greater diameter than body; latter cylindrical. Horn short, stout at base, tapering gently to a bifid tip, each arm of which bears a short white bristle. Colour of head and body green, horn black with the base and ventral surface green. (Second instar not recorded.)
In the third and fourth instars, head triangular, with a short process rising from the apex of each lobe. Body nearly cylindrical, of less diameter than head. Horn short, thick at base, tapering sharply to a point. Colour of head dark green with evenly-spaced paler-coloured tubercles. Colour of body, markings and tubercles as in the fifth instar, except that the subspiracular line of tubercles and the oblique lateral stripes on segments 6 to 10 are formed of very small-tubercles, and that the tubercles on dorsal and ventral surfaces of the horn are shiny black (Bell & Scott, 1937).
By the fifth instar, head rounded-triangular, very broad above the mouthparts, dorsal line hardly depressed. Apical head processes of earlier instars reduced to a large, low, rounded tubercle on the apex of each lobe. Clypeus about one-third length of head, and there is no false-clypeus. Labrum as broad as clypeus, narrowing frontad. Ligula not so broad as labrum. Surface of head shiny, covered with unevenly-spaced, large, low, rounded tubercles, these tubercles smaller and more widely spaced on vertex and cheek. Body segment 2 of greater diameter than head, its front margin raised into a sharp ridge. Rest of body nearly cylindrical (but the venter somewhat flattened when full-fed), tapering slightly to 12. Horn short, stout at base, tapering sharply to a point, slightly down-curved. Surface of body dull, with a shiny, small, rounded tubercle on each side of the dorsal line on the frontal ridge of segment 2. There are a pair of larger end more pointed tubercles, one above the other, below this tubercle. From the lower tubercle of the pair a continuous ridge, from which rounded tubercles rise, runs to the level of top of spiracle. There are smaller, widely spaced tubercles around the secondary rings of all the segments from 2 to 12; a larger tubercle being present on the subdorsal line of 3 and 4. There is a subspiracular line of large wartlike tubercles, each with three or four rounded points, starting from just below and behind the spiracle of 2 and meeting the front end of oblique lateral stripe on 6 at a sharp angle. The oblique lateral stripes on 5 to 11 formed of a ridge, broken by the junctions of the secondary rings into oblong tubercles. Horn covered with shiny pointed tubercles; anal flap edged with rounded tubercles (Bell & Scott, 1937).
In colour, head dark bluish-green, immaculate; the tubercle on the apex of each lobe yellow. Labrum, ligula and basal segment of antenna pale yellow; rest of antenna chestnut. Mandible pale chestnut, tip darker chestnut. Body green above the spiracular line, bluish-green below it, but segments 2 to 4 darker than the rest. Tubercles and ridge on front margin of 2 orange, remaining tubercles on 2 yellow. Those on the remaining segments pale yellow above the spiracular line and of the body-colour below it, the four larger tubercles on the subdorsal line of 3 and 4 nearly white. There is a subspiracular line of tubercles on 2 to 4. Oblique lateral stripes shiny white, those on 10 and 11 slightly more prominent than the rest. All oblique stripes are edged above with dark green, and each stripe runs on to the adjoining segment in front and behind, but not reaching the dorsum, the forward and backward extensions being less prominent than the median part. Horn dark bluish-green, with paler tubercles on the dorsal and ventral surfaces, and pale yellowish-green with larger tubercles of the same colour on the sides and on the apical quarter. Anal flap edged with yellow tubercles. Basal segments of true legs pale yellow-green on the outer face and black on the inner, second segment brown on the outer face and black on the inner, third segment chestnut. There is a black patch on the venter opposite the base of each leg. Prolegs and claspers of the body-colour, feet pale brown. Spiracles oval, pale yellow edged narrowly with green (Bell & Scott, 1937).
The larva is sluggish and deliberate in its movements. In the resting position the front of the body is raised slightly from the surface, the face parallel with the surface. In this position the subspiracular line of tubercles forms almost a straight line with the oblique lateral stripe on segment 5.
The larva has the same habit as some of those of the genus Clanis of remaining in the larval state for a long period after burying itself in the ground. In India, some larvae which went underground in September and October 1932 had not pupated by May 1933, and others did not pupate until June 1933. The moths emerged about a fortnight after actual pupation (Bell & Scott, 1937).
PUPA:
Larval hostplants. Unknown in China but recorded on Indigofera (Fabaceae) in Uttar Pradesh, India (Bell & Scott, 1937). According to Captain Lang, who first found the species, C. exusta also feeds on Populus (Butler, 1875), but this record is questionable and may be a captive rearing.
Unknown.
China: Xizang/Tibet (Zhangmu, 2200m; Nyalam, 2000m).
Occurs from northern Pakistan (Margalla Hills, 1000m; Kaghan Valley; Miandam, Khyber Pakhtunkhwa) (Rafi et al., 2014; Younus & Uddin, 2015; Haxaire, Gujjar & Saeed, 2017) and northwestern India (Bortolin et al., 1998; Alok Mahendroo, pers. comm. 2012; Bhuyan, Clark & Smetacek, 2019), eastward along the southern slopes of the Himalaya to central Nepal (Haruta, 1992), Bhutan (Irungbam & Irungbam, 2019), and neighbouring parts of Xizang/Tibet. (Records from Hubei, China, are erroneous, and are probably based on misidentified species of Clanis.)
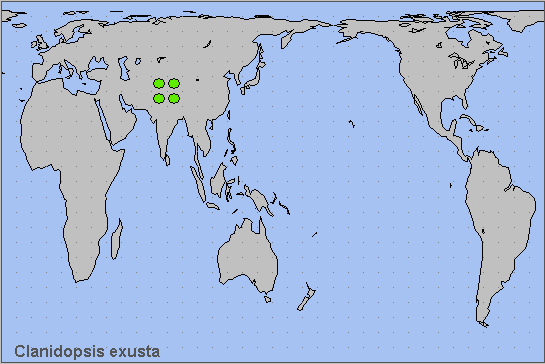
Holarctic; eastern Palaearctic region. Pleistocene refuge: Monocentric -- southern section of Sindian refuge.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list