![Male Cechetra bryki, Nepal, Kathmandu [Valley], Mt. Pulchouki [Phulchoki], 2770m [ca. 2500m], 16.vii.1990 [HOLOTYPE], leg. J. M. Cadiou. Photo: © The Trustees of the Natural History Museum, London (NHMUK) Male Cechetra bryki, Nepal, Kathmandu [Valley], Mt. Pulchouki [Phulchoki], 2770m [ca. 2500m], 16.vii.1990 [HOLOTYPE], leg. J. M. Cadiou. Photo: © The Trustees of the Natural History Museum, London (NHMUK)](c_bry_c1.jpg)
Cechetra bryki Ivshin & Krutov, 2018, Zootaxa 4450(1): 1-25. Type locality: Nepal, Kathmandu [Valley], Mt. Pulchouki [Phulchoki], 2770m [ca. 2500m], 16.vii.1990.
Synonym. Cechenena lineosa f. viridula Bryk, 1944 [invalid].
According to Ivshin & Krutov (2018), morphologically very similar to Cechetra scotti (Rothschild, 1920) and Cechetra lineosa (Walker, 1856), but has a brighter green background with less prominent diagonal lines on the forewing upperside. The forewing ground colour is green (may be yellowish-grey, olive-grey or brownish-green in old or discoloured specimens) without the gradient in the green tone observed in Cechetra lineosa. The diagonal lines of the forewing upperside are weakly differentiated from the ground colour, with some, particularly the most distal one, barely visible, unlike Cechetra subangustata (Rothschild, 1920) in which all eight dark green or brown diagonal lines are clearly visible. The outer edge of the black basal patch of the hindwing upperside extends along the veins. The female is similar to the male, but is larger with rounder forewings and with the black basal patch of the hindwing upperside with less pronounced sharp triangles extending along the veins.
The structures of the male genitalia are within the range of variation of both Cechetra lineosa and Cechetra scotti and are very different from those of Cechetra minor (Butler, 1875) and Cechetra pollux (Boisduval, [1875]). The uncus lacks an acute sclerotized hook ventrally; the sclerotized process of the harpe is elongate, almost the same width over the entire length; and the margin of the basal plate of the aedeagus armature is deeply denticulate with a large apical hook distally (the basal portion of this hook is angled at about 30° to the aedeagus main axis). In the male genitalia of the superficially similar but larger Cechetra subangustata, the uncus bears an acute tooth ventrally, the sclerotized process of the harpe is chunky, noticeably narrowing distally; and the aedeagus armature has a less denticulate plate and a short, small apical hook that is devoid of additional minute teeth on its surface (the basal portion of the hook is angled at about 45° to the aedeagus main axis). Generally, Cechetra bryki has thinner and more elongate male genital structures than Cechetra subangustata.
Examination of the 'type' of Cechetra lineosa f. viridula (Bryk, 1944) convinced Ivshin & Krutov (2018) that it is just a discoloured specimen of Cechetra bryki. Another moth, in NHMUK, was illustrated by d'Abrera ([1987]: pp. 205-206) as a 'green form' of Cechetra lineosa, but he did not give any locality data. Ivshin & Krutov (2018) examined this specimen, which proved to come from Sikkim, India, and found that it, too, is a specimen of Cechetra bryki. They included both these specimens in the paratype series of Cechetra bryki.


Ecologically, Cechetra bryki seems to belong to the equatorial cloud forest fauna and thus have a patchy distribution due to the patchy nature of this habitat (Ivshin & Krutov, 2018).
China: v-viii (Yunnan).
Currently, only one generation per year is known for Cechetra bryki, from the end of May until early August (Ivshin & Krutov, 2018).
OVUM: Unknown.
LARVA: Unknown.
PUPA: Unknown.
Larval hostplants. The larval hostplants of Cechetra bryki are currently unknown. The hostplants of the closely related Cechetra lineosa were listed by Bell & Scott (1937) as Impatiens, Saurauia, Polygonum and Vitis. All these are members of early succession plant communities, which is typical for most hawkmoths of subfamily Macroglossinae (Ivshin & Krutov, 2018).
China: Yunnan (Yuxi, Mt. Ailaoshan, 2300m; Xinping, Mt. Ailaoshan, 2300m).
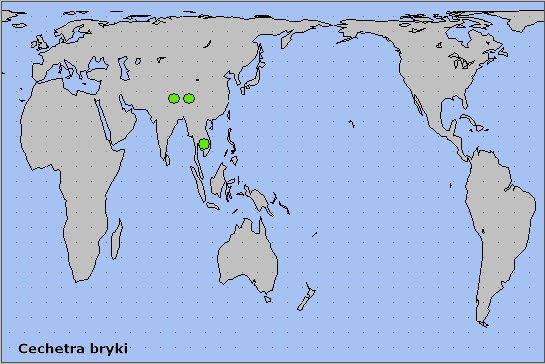
Nepal, Bhutan (Irungbam & Irungbam, 2019), northeast India, Myanmar/Burma, southwestern China (Yunnan), Laos and northern Vietnam (Ivshin & Krutov, 2018).
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list