Sphinx (Acherontia) styx Westwood, 1847, Cabinet oriental Ent.: [88], pl. 42, fig. 3. Type locality: "East Indies".
Synonym. Sphinx styx Westwood, 1847.
Synonym. Acherontia ariel Boisduval, 1875.
Synonym. Acherontia styx interrupta Closs, 1911.
Synonym. Acherontia styx obsoleta Schmidt, 1914.
Note. Separation of Acherontia styx medusa Moore from Acherontia styx styx in China is problematic because the two taxa appear to intergrade along a wide front. Specimens in the IZAS from Shanghai, Guangdong and Hong Kong are all typical Acherontia styx medusa, whereas those from Xizang/Tibet, Sichuan and Shaanxi are typical Acherontia styx styx. The intervening populations along this east-west divide are, to a greater or lesser extent, intermediate. Consequently, we do not allocate the following records to subspecies. Furthermore, Kitching & Spitzer (1995) stated that "specimens from Vietnam are somewhat intermediate between subspecies styx and medusa". It seems, therefore, that Acherontia styx medusa may just be a wet zone/season form.
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan.]
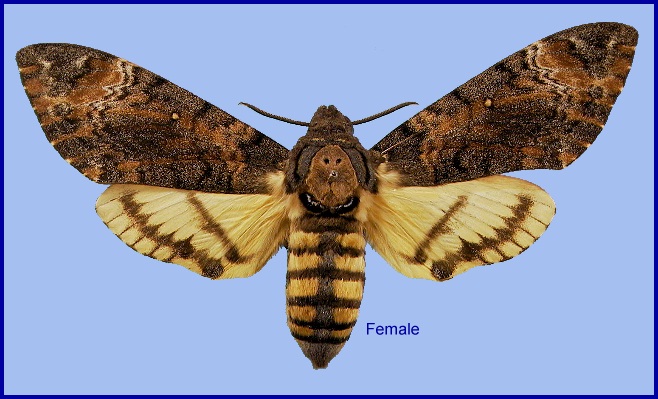
Wingspan: 89--130mm. Although individually variable in colour and pattern, forewing upperside generally differs from Acherontia styx medusa in possessing tawny russet streaks, and a similarly coloured patch distal to the greyish white discal lines. Forewing underside usually with a blackish cloud in the discal cell along the cubital vein proximal to the middle; discal third of the wing usually with much black scaling; two discal bands usually present and the trace of a third, the first variable in position, sometimes close to the discal cell, the second often indistinct or occasionally absent.
Hindwing upperside differs from Acherontia lachesis in having the basal half entirely yellow, as in Acherontia atropos, and lacking a large black mark; discal black band generally more proximal and straighter than in Acherontia atropos, reduced in width and length in many individuals; distal black band usually more deeply incised between the veins on the proximal edge, often separated into vein-streaks connected by diffuse black scaling. Hindwing underside with discal spot occasionally obliterated; in some individuals it is touched by the discal band.
In the male genitalia, harpe with ventral process almost vertical on the plane of the valve, its broader side nearly horizontal; the second process triangular, as in Acherontia atropos, its broader side dorso-ventral (vertical); both processes simple or indistinctly notched. In the female genitalia, ostium bursae without process, but with a mesial carina running proximad from the rim of the opening.
Differs from the European Acherontia atropos in having two discal bands on the underside of the forewing, instead of one, and usually no dark bands across the ventral surface of the abdomen. Also, on the forewing upperside subbasal line less curved distad in the cell than in Acherontia atropos; white or buffish basal and discal spaces between the black dentate lines generally less pronounced than in Acherontia atropos. The forewing discal spot (stigma) is orange; in Acherontia atropos it is usually white. The skull-like marking on the thorax is darker and there is a faint blue tornal dot enclosed by a black submarginal band on the hindwing upperside.


An avid robber of honey in bee hives (Pittaway, 1993); also, a fruit-piercing pest of yuzu (Citrus junos) in South Korea (Choi, Kim & Lim, 2000). Although not a regular migrant, limited numbers are found each year as far north as Heilongjiang. It favours hot, open, low-lying country, either under semi-cultivation.
Due to the difficulty of assigning many specimens to either Acherontia styx styx or Acherontia styx medusa, the below flight-times are a combination of both subspecies. (See "Global Distribution" for more details.)
China: iv (Jiangxi); vi (Guangdong; Jiangxi; Shandong; Hubei); vii (Hainan); viii (Hubei); 7-9.viii (Zhejiang); 24-31.viii (Shanghai); ix (Jiangxi; Shaanxi); 1.x (Zhejiang). Taiwan: 2.vii (Kaohsiung Hsien); 12.vii (Chiayi Hsien). South Korea: 3.viii. Japan: 26.vii (Shikoku); 12-23.viii (Shikoku; Honshu). Russia: 1.vii.2012 (Primorskiy Krai).
In both northeastern and southern China, there are two generations a year, with adults flying in May/June and August and larvae generally found from June until September.
Park et al. (1999) give late July until mid September as the flight period in Korea.
OVUM: Pale green, changing to yellowish green just before hatching. Oval (1.2 x 1.5mm), shiny and smooth. Laid singly on the under and upper surfaces of leaves on peripheral twigs, usually hatching three to five days later.
In India, the eggs of this species are often attacked by a hymenopterous parasite, which lays its eggs in or on those of the moth. Infected eggs become gradually mottled black and white as the larvae of the parasite develop. As many as twenty parasites may emerge from one egg (Bell & Scott, 1937).
LARVA: Full-fed 90--120mm. Trimorphic: green, yellow or brown.
The newly-hatched, 5mm-long larvae immediately consume their eggshells. At this stage they are 'frosty' yellowish green with a long, black, fork-tipped horn. In the second instar white lateral stripes and numerous small white tubercles appear. It is not until after the third moult that the final colours become apparent. Fully-grown larvae closely resemble those of Acherontia atropos except that the dark blue dorsal speckling is more pronounced on the anterior half of each abdominal segment, and the horn is less curved and lacks a reflexed tip.
In the green form, head dark green, with a broad, shiny black stripe down the cheek; labrum whitish with a black spot in the middle of each half; ligula whitish, the lobes streaked with black; basal segment of antenna white, middle segment white with black base, third segment pale red, mandible pale green with the tip broadly black. Segments 2 to 4 of body yellowish-green, but rest of body grass-green. Dorsum and sides of 5 to 11 with dark bluish dots along each secondary ring. There are seven sharply defined yellow oblique lateral stripes on 5 to 11, each stripe extending upwards and backwards to near the dorsal line of the segment behind, that on 11 extending backwards to the base of the horn; each stripe edged above with dark blue, sharply defined at the common edge but diffuse dorsad. Horn canary-yellow; true legs black; prolegs and claspers green; anal flap green edged with yellow. Spiracles oval, yellowish-white with the central slit black, the whole bordered with brownish-green (Bell & Scott, 1937).
In the yellow form, the green colour of head and body replaced by canary-yellow, but with the markings remaining the same.
In the brown form, head ochreous with the stripe dark brown. Body brown, with a broad black dorsal stripe onsegments 2 to 4 and below it a broad ochreous stripe. The subspiracular area dotted and streaked with brown; a brown oval marking on each side of the dorsum of 2. Oblique lateral stripes purple; horn ochreous, legs and prolegs black, claspers brown (Bell & Scott, 1937).
Larger larvae are lethargic and confine their strip-feeding to two or three shoots. At 40°C, growth is exceedingly rapid, ovum to pupa taking as little as 10 days.

PUPA: 50--60mm. Similar to, but paler than that of the European species Acherontia atropos. Antenna slightly longer than fore-leg. Cremaster stout, triangular, dorsal surface rugose, tip ending in two teeth, each bearing a bristle. However, unlike Acherontia atropos, rarely formed more than 10cm below the surface of the soil, in a smooth-sided chamber. The main overwintering stage. Very tolerant of extremes of moisture or dryness.

Larval hostplants. Recorded in China from Clerodendrum, Ligustrum, Lycopersicon, Sesamum, Solanum and various Fabaceae (Mell, 1922b; Yang, 1978; Li & Guo, 1990).
In Japan, recorded from Sesamum indicum and Solanum jasminoides.
Recorded in Korea on Solanum melongera, Solanum tuberosum, Capsicum annuum, Pisum sativum and Paulownia tomentosa (Park et al., 1999).
In Laos and Thailand, recorded from Duranta repens, Jasminum sambac, Solanum melongena and Solanum verbascifolium (Eitschberger & Ihle, 2008; 2010).
Elsewhere, most larval hostplants are Bignoniaceae, Fabaceae, Oleaceae, Pedaliaceae, Solanaceae and Verbenaceae, with occasional records from eleven other families.
In India, the larvae sometimes occur in such numbers as to cause serious damage to crops, such as Sesamum indicum (Bell & Scott, 1937). It is also a minor pest of the flowering ornamental Jasminum sambac, both in India (Davidson, 2023) and Thailand.
Ichneumonidae: Quandrus pepsoides (Smith, 1852); Trichogrammatidae: Trichogramma achaeae Nagaraja & Nagarkatti, 1970.
Due to the difficulty of assigning many specimens to either Acherontia styx styx or Acherontia styx medusa, the below distributions are a combination of both subspecies. (See "Global Distribution" for more details.)
China: Hebei; Beijing; Shandong (Jinan; Yantai); Shanxi (Taigu; Xiaxian; Jiexiu; Changzhi); Shaanxi (Yangling; Xunyang, 1380m); Henan; Jiangsu; Anhui (Mt. Huang Shan); Shanghai; Zhejiang (Ningpo; Tianmu Shan); Hubei (Changyang; Yichang; Mt. Guifeng, Macheng); Sichuan (Emei Shan; Gao Xian; Hongyuankun; Huili); Yunnan (Gaoligong Shan); Xizang/Tibet (Zhangmu, 2200m); Hunan; Jiangxi (Jiujiang; Nanchang); Guangdong (Foshan; Guangzhou; Shantou); Macau; Hong Kong; Guangxi; Hainan.
Taiwan: Chiayi Hsien (Alishan); Kaohsiung; Kaohsiung Hsien (Liukuei).
North Korea. South Hamgyong Province (Wonsan).
South Korea: Seoul; Kyonggi Province (Suwon; Gwangleung; Deokeun-ri; Cheonma-san; Asan Bay; Ganam-myun; Geumdang-ri); Kangwon Province (Seolak-san; Chiak-san; Shillim; Yangyang; Daeam-san); North Chungchong Province (Chupungryung; Minjuji-san); South Chungchong Province (Gongju; Gyeryong-san); North Cholla Province (Byunsan; Mujugucheondong; Jiri-san; Naebyun-san; Unjang-san; Muju); South Cholla Province (Baekam-san; Baekyang Temple; Naju; Wolchul-san; Jogye-san; Geumo-do; Gurye; Yeocheon; Haenam); North Kyongsang Province (Daegu; Sobaek-san; Gimcheon; Juwang-san; Naeyon-san; Seongju; Cheongdo); South Kyongsang Province (Baekun-san; Gibaek-san; Hamyang; Jinyang; Muhak-san; Jinju; Geoje-do; Goseong; Namhae; Milyang; Sacheon; Sancheong; Uiryong; Hadong; Hapcheon); Cheju Province (Cheju-do).
Japan: Honshu (Mt. Mikabo); Shikoku (Matsuyama; Kajigamori; Mononobe); Kyushu; Tsushima; Ryukyu Archipelago (Okinawa).
Russia: Primorskiy Krai (Valentin settlement area, Lazo District).
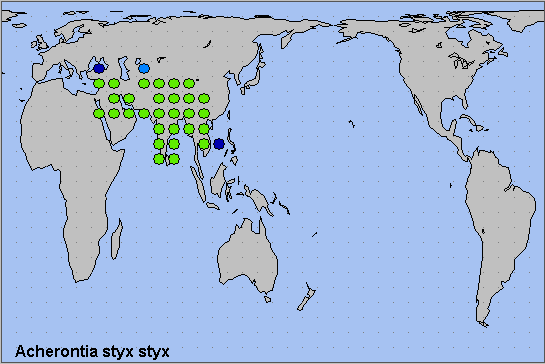
The core distributions of the two subspecies are as follows. Acherontia styx medusa occurs throughout eastern continental Asia, from northeastern China (to where it is a migrant) and Japan, south through eastern China and Vietnam (Le & Vu, 2024) to Peninsular Malaysia and peninsular Thailand. Also found throughout the islands of the Malay Archipelago, from Sumatra, Borneo and the Philippines, east to the Maluku Islands. Acherontia styx styx occurs from north-central and western China westward across northern Thailand, Burma/Myanmar, Bangladesh, India (Pathania, Sunita Sharma & Gill, 2014; Subhasish Arandhara, 2016), Nepal, Bhutan, Pakistan (Rafi et al., 2014; Humairah Hanif et al., 2016) and Iran to Saudi Arabia, Egypt, Israel and Iraq (Pittaway, 1993).
The 2012 record from the Russian Far East is of a single individual, most likely a vagrant (Dubatolov & Yakovlev, 2013).
Palaeotropical; Oriental region.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list