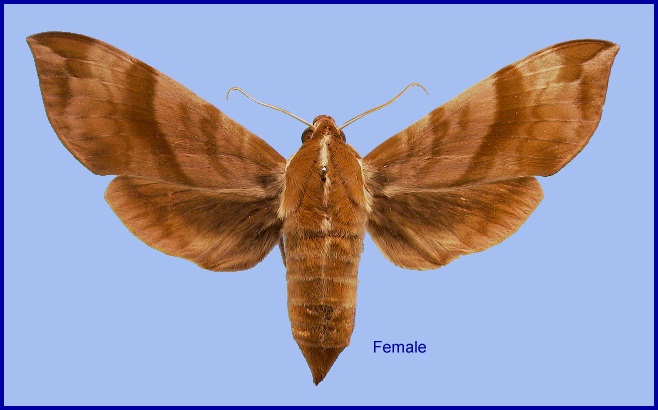
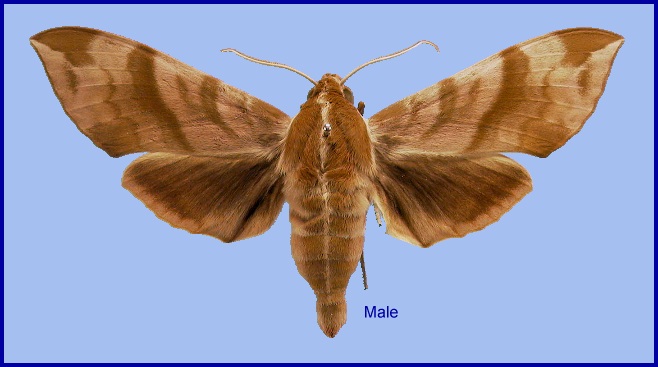
Ampelophaga rubiginosa Bremer & Grey, 1853, in Motschulsky (ed.), Etudes ent. 1: 61. Type locality: North China: <<vicinity of Beijing>>.
Synonym. Deilephila romanovi Staudinger, 1887.
Synonym. Ampelophaga romanovi (Staudinger, 1887).
Synonym. Acosmeryx iyenobu Holland, 1889.
Synonym. Ampelophaga rubiginosa alticola Mell, 1922.
Synonym. Ampelophaga rubiginosa hydrangeae Mell, 1922.
Synonym. Ampelophaga rubiginosa marginalis Matsumura, 1927.
Synonym. Ampelophaga rubiginosa submarginalis Matsumura, 1927.
Note. The population found in south-western China (Yunnan) and north-eastern India used to referred to as subspecies Ampelophaga rubiginosa harterti Rothschild, 1895 (with a red underside) [= Acosmerycoides harterti], whereas the paler, browner and less distinctly marked population found in northern India, Nepal and Pakistan was named subspecies Ampelophaga rubiginosa fasciosa Moore, 1888. However, recent findings have demonstrated that these 'subspecies' are good species. The small greyish Taiwanese population is less distinct and warrants subspecific status -- Ampelophaga rubiginosa myosotis Kitching & Cadiou, 2000.
Note. An eastern Palaearctic species which is almost indistinguishable from the western Palaearctic Clarina Tutt, 1903 at every stage of development. This close relationship was noted by Kawahara et al. (2009).
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan as well as Moths of the southern Shikoku, Japan.]
Wingspan: 72--100mm. Most individuals are on the upperside a dark reddish-brown with a hint of purple. The forewings are crossed by several darker oblique brown bands, whereas the hindwings are an almost uniform fuscous, although there is a hint of a submarginal dark band behind the white fringe. Hindwing veins M2 and M3 much further apart than M3 and Cu1. A very characteristic feature of this species is a white or cream dorsal line which runs from the head to the tip of the abdomen. The underside is salmon-buff or clayish, each wing with two faint transverse lines. There is no comb on the mid- and hind tarsus (Bell and Scott, 1937).
In the male genitalia, uncus truncate, weakly sinuate in distal view. Harpe regularly ladle-shaped. Phallus with a long left process that is irregularly and sparsely dentate and notched at proximal edge; the right process short, usually simple, but sometimes with one or two minute teeth.
In some individuals/forms the uppersides are considerably paler and olive-brown (D'Abrera, 1986), others have very faint markings and, in some, the undersides are redder.
Similar to Dahira rubiginosa Moore, 1888 and Ampelophaga khasiana Rothschild, 1895. The former has no pale dorsal stripe, the latter has more falcate forewings.



Open forest, parkland, scrub, suburban gardens, and cultivated vineyards, in other words, this common partial migrant occurs almost anywhere where its hosplants grow.
In the Russian Far East, females are active between 23.10h and 01.50h, the males from 00.20h until 04.20h. A lowland species of mixed and pure deciduous woodland characterized by Quercus mongolica (Izerskiy, 1999b).
The adults are usually never very common, occurring at between 1200m and 2200m altitude in the himalayan foothills of northern India (Bell and Scott, 1937) and Tibet (Wang, 1988). In Nepal it is met with from May until September (Haruta, 1992); they rarely come to light or flowers.


China: i (Yunnan); ii-v (Shanghai); iv-vi (Jiangxi); v (Sichuan; Yunnan); vi (Shanghai; Yunnan; Beijing; Sichuan); 20-26.vii (Shaanxi); vii (Guangdong; Heilongjiang; Liaoning; Guangxi; Shanghai); viii (Hubei; Shandong; Fujian; Sichuan; Shanghai); 24.ix (Shanghai). South Korea: 26.vii ([unstated locality]). Japan: 9-13.vi (Shikoku); 19.vi-27.vi (Honshu); 25.vi-29.viii (Hokkaido); 5-30.vii (Honshu); 30.vii (Tsushima); viii-ix (Honshu). Russia: 16.vi-22.vii (Khabarovskiy Krai); 5-28.vii (Primorskiy Krai); 8-9.viii (Kurile Islands); 23.viii-11.ix (Primorskiy Krai).
There is one generation per year in north-eastern China, with adults in June-August (Yang, 1978). Farther south there may be up to three broods, e.g. in Jiangxi (Chu et al., 1979). In Beijing, where two generations are normal, larvae are met with in June/July and August/September (Yang, 1978). In Shanghai, adults can be found from February until October (Pittaway & Kitching, 2000).
Park et al. (1999) give early May until early August as the flight period in Korea.
OVUM: Pale green, almost spherical (1.36 x 1.42mm), shiny and smooth.
LARVA: Full-fed 69--95mm long, 16mm wide; horn 13 mm. Dichromatic: green or reddish-brown. According to Bell and Scott (1937), in the first instar the pale yellow larva has a black horn with a forked tip, cylindrical body and round head. After the first moult the body elongates, becomes green, and the horn acquires an orange base. In the third instar a transverse row of small yellow tubercles appears on each secondary body ring and the long, straight horn becomes reddish with black tubercles. In the next instar the first five body segments enlarge progressively in diameter, although the rest of the body remains cylindrical. The entire yellowish-green body and yellow-cheeked head become covered with fine yellow tubercles. Superimposed upon this is a yellow dorso-lateral line edged above with reddish-brown, which extends from the horn to segment two. There is a similar, but narrower yellow line each side of the dorsal heart-line. On abdominal segments 4 to 8 there are indistinct oblique side stripes made up of enlarged tubercles, these pointing in the opposite direction to those found on most sphingid larvae. The horn is still red with black tubercles. For most larvae this colour form persists into the final instar, with the yellowish-green body colour becoming more glaucous below the dorso-lateral line. The horn becomes greenish, curved and stout, with a blunt point. However, some larvae become predominantly orange-brown in the final instar rather than green, i.e. all green pigmentation (but not the yellow and white) is replaced by shades of reddish-brown. Both forms turn pale brown prior to pupation.
The larvae are mainly nocturnal and prefer younger leaves, often stripping growing shoots, particularly in the final instar. In the hills to the west of Beijing, full-grown larvae can often be found fully exposed on almost completely bare and unsupported leading shoots of wild grapes scrambling over shrubs (Pittaway, pers. obs. 2003).
Walls and fences overgrown with Parthenocissus are a favourite in Beijing, China (Pittaway & Kitching, 2000); larvae were common on this plant on 8.vii.1995 and during late August 2003 (Pittaway & Kitching, 2000; Pittaway, pers. obs. 2003).








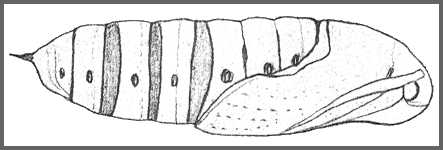
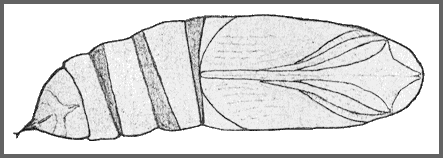
PUPA: 43--60mm long, 15mm wide. Stout, elongate-ovoid in shape. Ochreous in colour, speckled with black, with a distinct black spot on the front of the slightly projecting head. The tongue-case is not produced and lies flush with and between the wing-cases (Bell and Scott, 1937). The abdominal segments are a richer brown, finely punctate, with black spiracles. Cremaster conical, narrow, smooth, and terminating in a sharp point. As in Acosmeryx, the posterior margin of abdominal segment 8 is deeply undercut, overlapping segment 9. Formed in a loose brown cocoon among leaves on the ground. The overwintering stage



Larval hostplants. In China, mostly on Vitaceae (Causonis, Parthenocissus, Vitis) (Oberthür, 1886; Mell, 1922b; Yang, 1978; Chu et al., 1979), with a single record from Hydrangea paniculata in Guangdong (Mell, 1922b), and Hydrangea involucrata from Japan (Murase, 2001). Records on Convolvulus (Oberthür, 1886) and Vitex (Verbenaceae) (Chu & Wang, 1980) are erroneous. Bell & Scott (1937) also recorded Ampelophaga rubiginosa in the Khasi Hills on Saurauia (Actinidiaceae), and it has been found on Actinidia arguta (Siebold & Zucc.) Planch. ex Miq. in Japan (Hayashi, 2006). [Note: It appears that Causonis has been recorded as the only host in Taiwan (Lin, 1987).]
Recorded in Korea on Vitis amurensis, Vitis flexuosa, Vitis vinifera, Parthenocissus tricuspidata, Salix, Prunus serrulata var. spontanea and Malus pumila (Park et al., 1999). The last three hosts are so unusual as to warrent further study.
![Causonis japonica [syn. Cayratia japonica], Hangzhou, Zhejiang, China. Photo: © Tony Pittaway. Causonis japonica [syn. Cayratia japonica], Hangzhou, Zhejiang, China. Photo: © Tony Pittaway.](cay_jap.jpg)
Unknown.
China: Heilongjiang (Lalin; Zhaodong; Lesser Khingan Mountains, Fengling Forest; ??Ertzendziandzy; Liangshuigou; Yabuli Skiing Tourism Resort); Jilin (Tonghua); Liaoning (Maquanzixiang, Fushun County; Huanren; Changhai, Dachangshan Island); Hebei; Beijing (Haidian; Badaling; Botanical Garden; Baihua Shan); Tianjin; Shandong (Tsilan; Weihai; Yantai; Jinan; Qingdao); Shanxi (Taigu; Taiyuan; Zuoquan; Lishi; Xiaxian); Shaanxi (Hua Shan; Louguantai Forest Park; Xi'an; Taibaishan); Ningxia; Henan (Tonghecun; Zhumadian); Jiangsu (Nanjing; Longtan; Baohuashan, Zhenjiang City; Yangzhou); Anhui (Mt. Huang Shan; Anqing; Hefei); Shanghai; Zhejiang (Tianmu Shan; Hangzhou; Kuocang Mountain Nature Reserve); Hubei (Badong; Hefeng; Lichuan; Xuan'en; Yichang; Wuhan; Xianning); Sichuan (Chengdu; nr. Luding; nr. Mianning, 5000'; Emei Shan; Kangding, 7500'; Aba/Ngawa, 2450m; Yinjing); Chongqing; Yunnan (Dali; ??Bahand; Lijiang; Yanmen; nr. Yingjiang, 1180m; Mengla County, Mt. Leigongyan, 2000m; Gaoligong Shan; Simao/Pu'er; Yangbi Yi Autonomous County, Dali); Xizang/Tibet (Mutu, Namjagbarwa area, 2000m); Guizhou (Tuanlongcun; Qiandongnan Miao and Dong Autonomous Prefecture; Qiannan Buyei and Miao Autonomous Prefecture); Hunan (Daweishan; Changsha); Jiangxi (Jiujiang; Nanchang; Wanzai; Fuzhou); Fujian (Longqi Shan; Fuzhou; Guangze; Sanming); Guangdong (Wanzishan, Deqing); Hong Kong; Guangxi (Jiajiu Shan, Bamian; Damingshan; Chongzuo); Hainan (Baisha).
North Korea: Kangwon Province (Keumgang-san); South Pyongan Province (Pyongyang); South Hamgyong Province (Seokwang Temple); North Hamgyong Province (Gyungsung; Nanam).
South Korea: Baengnyeong-do & Daecheong-do; Seoul; Kyonggi Province; Kangwon Province (Jang-san); North Chungchong Province; South Chungchong Province; North Cholla Province; South Cholla Province; North Kyongsang Province; South Kyongsang Province; Cheju Province
Japan: Hokkaido (Hakodate; Sapporo; Shari): Honshu (Mukoyama; Nikko; Oiwake; Tokei-ji; Yokohama; Gifu; Nashimoto; Tokyo; Mikaboyama, 750m; Kamasawa; Sayama; Akashina; Oki Islands): Shikoku (Shioemachi; Yamashiro): Kyushu (north): Tsushima (Sasuna).
Russia: Amurskaya (Uril area); Yevreyskaya (Bastak); Khabarovskiy Krai (Slavyanka; Khabarovsk; Bolshekhekhtsyrskii Nature Reserve, Khabarovsk suburbs; Komsomolsk-na-Amure; Pivan); Primorskiy Krai (Askold Island; Jankowski Peninsula; Khasan; Primorskiy; Narva; Kedrovaya Pad Nature Reserve; Andreevka; Novovladimirovka; Ussuriysk; Vityaz Bay; Anisimovka; near Zanadvorovka; Barabash; Dalnegorsk; Kravtsovka; Tygrovyi); Kurile Islands (Kunashir Island).
The traditional distribution has this species occurring from northeastern Afghanistan, east along the Himalayan foothills of Pakistan (Rafi et al., 2014), India (Hmar et al., 2023; Chakraborty, Chakraborty, Biswas, Chakraborty & Deb, 2024), Bhutan (Irungbam & Irungbam, 2019) and Nepal, through southern and eastern China to the Korean Peninsula, the Russian Far East (Primorskiy Krai and Kunashir Island (Spitsyna & Spitsyn, 2023)) and Japan; also southeast through Burma/Myanmar, Thailand, Laos and Vietnam (Le & Vu, 2024) to Peninsular Malaysia and northern Sumatra. However, this range is now known to encompass three distinct species.
We consider the mapping of a single female by Danner et al. (1998) (as 'Ampelophaga rubiginosa fasciosa Moore, 1888') in the Kopet Dag of southern Turkmenistan to be erroneous. The locality of this specimen is actually 'Turkestan', an imprecise designation that can be applied equally to much of Central Asia.
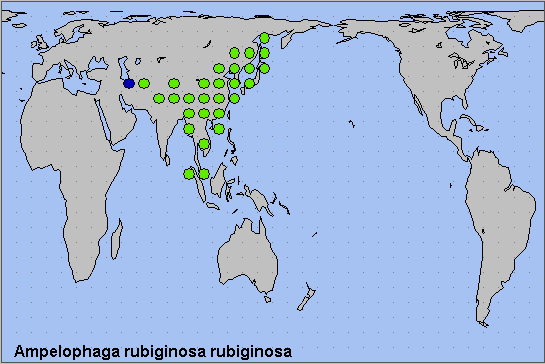
Holarctic; eastern Palaearctic region.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list