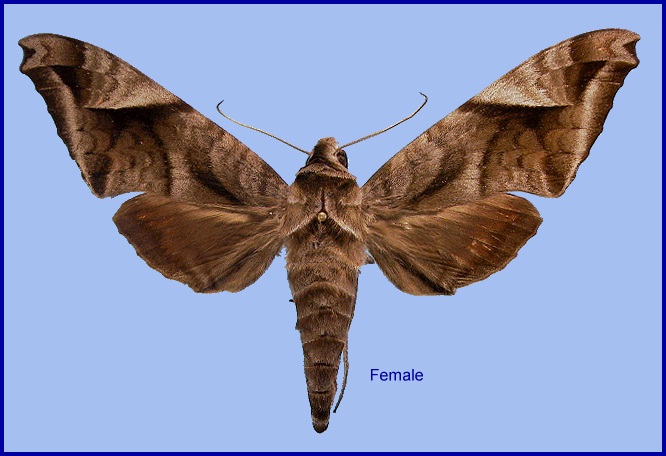
Acosmeryx metanaga Butler, 1879, Annals and Magazine of Natural History (5)4: 350. Type locality: [Japan,] Yokohama.
Synonym. Acosmeryx naga naganana Zolotuhin & Yevdoshenko, 2019.
Note. Synonymized with Acosmeryx naga by Rothschild & Jordan, 1903, Novit. zool. 9 (suppl.): 529. Implicitly raised to a subspecies of Acosmeryx naga by Zolotuhin & Yevdoshenko, 2019, Hawkmoths of Russia and adjacent territories: 310, 448.
Note. DNA barcode data in BOLD supports the division of Acosmeryx naga into two subspecies, represented by BINs ACF4345 and AAB5314. Acosmeryx naga naga comprises all populations from Uzbekistan and Tajikistan to Thailand and Vietnam (and so includes pale moths formally assigned to Acosmeryx naga hissarica). The second, namely Acosmeryx naga metanaga Butler, 1879, comprises the populations from central, eastern and northeastern China, Taiwan, Korea and the Russian Far East (and so includes Acosmeryx naga naganana of Zolotuhin & Yevdoshenko (2019)). Consistent morphological differences between the two subspecies are difficult to determine but, in general, Acosmeryx naga metanaga is smaller and has a less contrasted forewing upperside pattern (Ian Kitching/Sphingidae Taxonomic Inventory, pers. comm. 2020). The outer margin of the forewing is generally less crenulate too, although this character may be under some environmental influence as specimens from higher and colder localities tend to be more crenulate (Jean Haxaire, pers. comm. 2020).
Zolotuhin & Yevdoshenko (2019) (STI 22079) described Acosmeryx naga naganana based on a holotype and five paratypes from Taiwan (Miaoli County, 21km E Tungshih [Dongshi]). Despite the name, Acosmeryx naga naganana was said to be a large subspecies. In the description it was said to differ from the nominotypical subspecies by the smooth external forewing margin. However, this character may be under some environmental influence as specimens from higher and colder localities tend to be more crenulate (Jean Haxaire, pers. comm. 2020) and so is not useful for distinguishing subspecies. Acosmeryx naga naganana was distinguished from the Japanese Acosmeryx naga metanaga by the absence of a brownish or reddish tint to the ground colour, the presence of distinct dark triangles on the abdominal upperside, an S-shaped (rather than straight) pale marginal band on the forewing upperside, and an 'almost straight not angled in costal zone upper part of dark postmedial notch'. However, examination of the long series of Acosmeryx naga from Taiwan and Japan in the NHMUK has shown that none of these characters is consistent. With regard to the male genitalia, Zolotuhin & Yevdoshenko (2019) (STI 22079) characterized those of Acosmeryx naga naganana as 'valvae egg-shaped (ovoid) not parallel-sided as in most other subspecies, valvar appendix [harpe] more compact and regularly dentate on the inner surface, phallus a bit shorter but with distinct apical spur which is strongly curved and lance-shaped'. Zolotuhin & Yevdoshenko (2019) (STI 22079) illustrated only the male genitalia and phallus of the holotype of Acosmeryx naga naganana and a specimen from Kunashir (Kurile Islands), together with just the phallus of a specimen from North India. The purported diagnostic features of the valve shape are inconsistent in that the shapes are different between the left and right, which is probably due to distortion when the genitalia were flattened. The valves of the holotype of Acosmeryx naga naganana are indeed more rounded than the left valve of the Kunashir specimen, but less rounded than its right valve. The shapes of the harpe are well within the range of individual variation of Acosmeryx naga and the reason that the apical transverse process of the phallus appears to have a diagnostic 'strongly curved and lance-shaped apical spur' is that this process has been lost from the other two illustrated preparations. Furthermore, the minimum DNA barcode divergence between samples from Japan and Taiwan is only 1.082%, less than the 1.186% maximum divergence within Acosmeryx naga naga (excluding the Thailand and Vietnam samples), and all samples of Acosmeryx naga from Japan, Taiwan and mainland China cluster in a single BIN, AAB5314. Consequently, there is no justification for retaining Acosmeryx naga naganana as a subspecies separate from Acosmeryx naga metanaga (Ian Kitching/Sphingidae Taxonomic Inventory, pers. comm. 2020).
[Further details on this species in Japan, as well as photos of many stages, can be found on Digital Moths of Japan as well as Moths of the southern Shikoku, Japan.]
Wingspan: 86--112mm. Apart from Acosmeryx purus Kudo, Nakao & Kitching, 2014, the most conspicuously marked species of the genus, easily distinguished by the pattern of its forewing. Forewing upperside with a brown discal band extending from the costa towards the middle of the distal margin, sharply defined anteriorly, the triangular area anterior to it grey; a well-defined, grey submarginal band running more or less straight from vein Rs4 to the tip of 1A+2A.
In the male genitalia, gnathos parallel-sided. Harpe with process quite acute distally, resembling a hand with the thumb lying alongside the forefinger, with the other fingers curved back and upwards. Phallus with left lobe shorter than in all other species of Acosmeryx. In the female genitalia, ostial plate similar to that in Acosmeryx anceus anceus.




China: 13.iii (Yunnan); 29.iv (Beijing); v (Zhejiang; Guangdong; Yunnan); vi (Yunnan; Hainan; Beijing); vii (Xizang/Tibet). Taiwan: 19.ii (Taoyuan Hsien); iii-v (Kaohsiung Hsien); vi (Hualien Hsien). North Korea: 15-29.v (North Pyongan Province). Japan: 30.iii (Kyushu); 25.iv (Honshu); 3-31.v (Honshu); 2.vi (Shikoku); 17.vi (Honshu); 18.vi (Hokkaido); 20.vi (Ryukyu Archipelago); 26.v-26.vi (Hokkaido). Russia: 9-10.vi (Yevreyskaya); 1-20.vii (Primorskiy Krai; Kurile Islands).
In northern China there is one generation per year, with adults flying between April and June (Yang, 1978).
Park et al. (1999) give early May until mid August as the flight period in Korea.
OVUM: A deep rich green, becoming whitish before hatching, almost spherical, smooth (Bell & Scott, 1937).
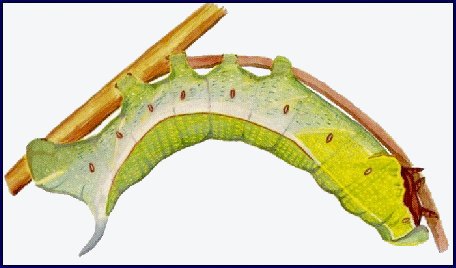
LARVA: Full-fed 80--100mm. Dichromatic: green or brown. According to Bell & Scott (1937), in the first instar, the head and body are green, the horn black, long and straight. In the second instar, thoracic segment 1 is as narrow as the head, with the body tapering sharply anteriorly from the first abdominal segment. The black horn is still long and straight, but reddish at the base and with a white tip. The head and anal segments are yellow, the body green dotted with white, with the spiracle of abdominal segment 1 surrounded by a black spot. A yellow dorso-lateral stripe runs from the head to the horn. This colour pattern remains the same for the next two instars, but a lateral flange develops on the third thoracic and first abdominal segments and a yellow ventro-lateral stripe appears on the thoracic segments and abdominal segment 1. In the final instar of the green form, the head is grass-green, with a narrow, pale yellowish dorso-lateral stripe and a broader stripe of the same colour separating face from cheek; thoracic segments 1 and 2 grass-green with short darker stripes; the rest of the body bluish green, mottled with yellow above the dorso-lateral stripe and pale greyish blue below. The dorso-lateral stripe is narrow and white on thoracic segments 1 and 2, broader and pale yellow on thoracic segment 3 and abdominal segment 1, then white to the base of the horn, and edged narrowly above with orange on segments 3--7. The narrow, white ventro-lateral stripe on thoracic segments 1 and 2 becomes broad and yellow as it outlines the flange before turning upwards on abdominal segment 1 to form an oblique stripe. There are also pale yellow, oblique lateral stripes on segments 1--7. The horn is lilac-grey dotted with purple. There are dark purple patches on the body above the bases of the legs, increasing in size caudally, and extending along the lower edge of the flange on thoracic segment 3 and abdominal segment 1. Upper part of prolegs bluish, the lower part pale yellow; feet brown, anal clasper bluish, anal flap edged broadly with pale yellow. Spiracles deep orange. In the brown form the green pigmentation is replaced by pinkish brown. The whole body is smooth and moderately shiny.
At rest on the underside of a leaf or stem, the larva throws back its head and anterior segments in a sharp curve, the head held so that the face is in the same plane as the dorsum of thoracic segment 1. The thoracic legs are pressed close to the body and the flange is dilated laterally. Alarmed larvae withdraw their heads and first two thoracic segments into segment 3; thoracic segment 3 and abdominal segment 1 are puffed out and the flanges further dilated.



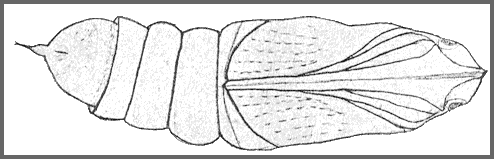
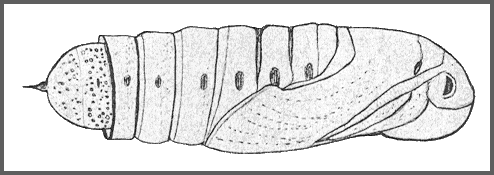
PUPA: 44--55mm long, 15mm wide. Very similar in shape to Clarina but colour of dorsum dark brown, sides and venter paler brown, abdomen marked with short darker stripes and pits dark brown. There is a pale brown patch in front of the eye; hind bevels of segments 8 to 11 dark brown; spiracles black ringed by dark brown; cremaster nearly black. Shape as in other Acosmeryx pupae, with the hind margin of segment 11 deeply undercut. Surface shiny, shallowly but coarsely pitted on segments 4 to 7 and 12 to 14. Segment 2 is finely, irregularly, longitudinally striate. Segment 8 with a patch behind the spiracle, finely, regularly, transversely striate. Front bevels of 9 to 11 finely pitted; rest of pupa smooth. Spiracle of segment 2 covered by a narrow transverse lobe projecting from the front margin of 3, the front edge of the lobe sharply raised. The remaining spiracles are shortly oval, flush, the edges of the: central slit raised. Cremaster small (2mm), basal half bulbous, distal half a cylindrical shaft ending in two small hooks. Dorsal surface of basal half honeycombed, shaft smooth (Bell & Scott, 1937).
Formed in a slight cocoon on the ground. Very sensitive to desiccation (Shchetkin, 1956), but highly tolerant of moisture. The overwintering stage.


Larval hostplants. Various species of Vitis, Cayratia and Ampelopsis (Vitaceae), such as Vitis amurensis, Ampelopsis brevipedunculata and Ampelopsis heterophylla in the Russian Far East, as well as Actinidia arguta (Siebold & Zucc.) Planch. ex Miq. (Vyacheslav Ivonin & Yanina Ivonina, pers. comm. 2022). In Shanxi also recorded from Actinidia chinensis (Li & Guo, 1990).
Recorded on Taiwan on Ampelopsis brevipedunculata, Ampelopsis cantoniensis and Saurauia tristyla.
Recorded in Korea on Ampelopsis brevipedunculata, Actinidia arguta and Vitis vinifera (Vitaceae) (Park et al., 1999).
Unknown.
China: Liaoning (Changhai, Dachangshan Island); Hebei; Beijing (Baihua Shan; Badaling National Forest Park); Shanxi (Taiyuan; Xiaxian); Shaanxi; Henan (Mt. Niuxinduo, Luoyang, 1763m); Anhui (Mt. Huang Shan; Waizanying); Zhejiang (Tianmu Shan; Kuocang Mountain Nature Reserve; Zhongwuli); Hubei (Badong; Wuhan); Sichuan (Pengshui; Wolong National Nature Reserve); Chongqing (Fengjie); Yunnan (nr. Yingjiang, 1180-2080m; Gaoligong Shan; Hongha; Malipo County, Wenshan Zhuang and Miao Autonomous Prefecture, 1860m; Dali); Guizhou (Jiucai Ling; Xinzhaidashan, Zhijin County, 1000-2000m); Xizang/Tibet (Mutu, Namjagbarwa region, 850m; Zhangmu, 2200m; Langmai, Gonjo County (30°45'N, 98°52'E)); Hunan (Dayong); Guangdong (Nanling National Forest Park, 1100m (Morishita & Kishida, 2000)); Hainan (Sanya, Mt. Jianfengling; Duowen Ling, nr Lingao; Longhushan, Wenchang City).
Taiwan: Hualien Hsien (Taroko National Park); Kaohsiung Hsien (Shanping; nr. Tuona, 15km SE of Shanping, 1050m); Taipei Hsien (Fushan); Taipei (Shinshan Dream Lake, Sih-ji); Nantou Hsien (Lianhuachih); Taoyuan Hsien (Taoyuan City).
North Korea: North Pyongan Province (Chonma County, Chonma-san).
South Korea: Baengnyeong-do & Daecheong-do; Seoul; Kyonggi Province; Kangwon Province (Jang-san); North Chungchong Province; South Chungchong Province; North Cholla Province; South Cholla Province; North Kyongsang Province (Ulleung-do); South Kyongsang Province; Cheju Province
Japan: Hokkaido (Hakodate; Sapporo; Sounkyo); Honshu (Tokyo; Kiyosato, 1300m; Mikaboyama, 750m; Oki Islands); Shikoku (Saijo); Kyushu (Nishinakama); Ryukyu Archipelago (Okinawa; Miyakoshima; Ishigakijima).
Russia: Amurskaya (Blagoveshchensk area); Yevreyskaya (Bastak Nature Reserve); Khabarovskiy Krai (Bolshekhekhtsyrskii Nature Reserve); Primorskiy Krai (Khasan; Lazovskiy nature reserve; near Zanadvorovka; 20 km SE of Ussuriisk; Kravtsovka; Anisimovka; Krounovka; Spassk area; Shkotovsky area); Kurile Islands (Kunashir Island).
As subsp. metanaga, from southern, central and eastern China (Bell & Scott, 1937) to Taiwan, Korea, Japan (D'Abrera, 1986) and the Kurile Islands (Rybalkin & Yakovlev, 2017). Several migrants had been reported from the Russian Far East since 2002-2003 (Beljaev, 2003), and it has now established a resident population in the southern Primorskiy Krai (Dubatolov, pers. comm. 2013; Koshkin et al., 2021). Also recently recorded from Ishigakijima, the main island of the Yaeyama Islands in the Ryukyu Archipelago, Japan (Kishida, 2022).
As subsp. naga, from southern Tajikistan (Shchetkin, 1949; Shchetkin, 1956; Derzhavets, 1984), Uzbekistan and Afghanistan (Ebert, 1969; Daniel, 1971; C. M. Naumann, pers. comm.) across the Himalayan foothills of Pakistan (Rafi et al., 2014; Haxaire, Gujjar & Saeed, 2017), India (Khan & Raina, 2017; Kaleka, Kaur & Singh, 2020; Jatishwor Irungbam & Fric, 2021), Nepal (Haruta, 1992) and Bhutan (Dierl, 1975; Irungbam & Irungbam, 2019) to northern Burma/Myanmar, Thailand, Laos, Vietnam (Le & Vu, 2024) and, maybe, Peninsular Malaysia.
[It should be noted that some of the individuals of this subspecies recorded from southern China and northern Vietnam may, in fact, be the newly-described Acosmeryx purus Kudo, Nakao & Kitching, 2014.]
Holarctic; eastern Palaearctic region. Pleistocene refuge: Monocentric -- Sinopacific refugium.
 Return to Sphingidae of the Eastern Palaearctic species list
Return to Sphingidae of the Eastern Palaearctic species list